Abstract
Aim:
To investigate the mechanism of chlorogenic acid (CA)-induced anaphylactoid reactions.
Methods:
Degranulation of peritoneal mast cells was assayed by using alcian blue staining in guinea pigs, and the degranulation index (DI) was calculated. CA-induced degranulation of RBL-2H3 cells was also observed and assayed using light microscopy, transmission electron microscopy, flow cytometry, and β-hexosaminidase release.
Results:
CA 0.2, 1.0, and 5.0 mmol/L was able to promote degranulation of peritoneal mast cells in guinea pigs in vitro, but it did not increase the degranulation of peritoneal mast cells in CA-sensitized guinea pigs compared with control (P>0.05). Treatment with CA 0.2, 1.0, and 5.0 mmol/L for 30, 60, and 120 min induced degranulation in RBL-2H3 cells in a dose- and time-dependent manner (P<0.01). Under transmission electron microscope typical characteristics of degranulation, including migration of granular vesicles toward the plasma membrane and integration combined with exocytosis, were observed, after CA or C48/80 treatment. Fluorescent microscopy and flow cytometric analysis showed that CA induced concentration-dependent translocation of phosphatidylserine in RBL-2H3 cells. β-hexosaminidase release in RBL-2H3 cells was significantly increased after incubation with 1 mmol/L CA for 60 min and 5 mmol/L CA for 30 min (P<0.01).
Conclusion:
CA induces degranulation of peritoneal mast cells and RBL-2H3 cells in guinea pigs, which might be one of the mechanisms of the generation of anaphylactoid reactions induced by CA.
Similar content being viewed by others
Introduction
Chlorogenic acid (CA) is widely distributed in the plant kingdom. It is a secondary metabolite of phenylpropanoid, which is produced through the shikimate pathway in the aerobic respiration of plants. Most of the heat-clearing and detoxifying Chinese medicines contain CA, and CA is also commonly used as the characteristic marker of quality control for Traditional Chinese Medicine (TCM) because of its antibacterial, detoxifying, antiphlogistic and cholagogic activities. Some scientists believe that CA is an allergen and, thus, contributes to the high incidence of anaphylactic reactions to intravenous heat-clearing and detoxifying Chinese traditional medicines1, 2, 3. However, there is no direct evidence to support the notion that CA can cause allergic responses4. Moreover, in contrast to the classic mechanisms of allergic reactions, most reported adverse effects from TCM injections occur at the first exposure to CA, indicating that no allergic sensitization is involved. These adverse effects also correlate with dose and concentration of CA. Therefore, it is necessary to clarify whether the observed adverse effects are anaphylactic or whether they are anaphylactoid reactions induced by CA. In our preliminary study, anaphylactoid reactions were observed when guinea pigs were injected with 2.0 mL of CA (2%); symptoms and the degree of reaction were not associated with sensitization, and the results of passive cutaneous allergy test was negative. In another study, anaphylactoid symptoms (eg, trembling, salivation, tears and even vomiting) were observed during the first injection of CA in Beagle dogs. Therefore, it has been assumed that CA may lead to anaphylactoid reactions. In this study, we used guinea pig peritoneal mast cells and the rat leukemic basophilic RBL-2H3 mast cell line to investigate the possible mechanisms of CA-induced anaphylactoid reactions in vitro.
Materials and methods
Animals
Male Hartley guinea pigs were purchased from Jiangnan Huishan Laboratory Animal Corporation (Wuxi, China) at 250–350 g weight and 10 weeks of age. All animals were housed with a 12 h light/12 h dark cycle at 22 °C and 55%±5% relative humidity. Food (from Zhejiang Science Animal Center, China) and tap water were provided ad libitum during an acclimation period of at least 10 days. All experiments were carried out according to the guidelines of China for the care and use of laboratory animals.
Cells
RBL-2H3 cells were obtained from the cell bank of the Chinese Academy of Sciences. Cells were cultured in Dulbecco's modified Eagle's medium (Gibco, Grand Island, USA) containing 10% heat-inactivated fetal bovine serum and 40 U/mL gentamicin in a humidified atmosphere of 5% CO2 at 37 °C.
Reagents
A CA standard (in vitro test) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). CA (in vivo test, >98%) was purchased from Guanghan Biological Products Co, Ltd (Sichuan, China). Compound 48/80 (C48/80) was purchased from Sigma (St Louis, USA). Trypsin was purchased from Gibco (Grand Island, USA). The annexin-V Fluos staining kit was purchased from Roche (Indianapolis, USA). Percoll and p-nitrophenyl-N-acetyl-β-D-glucosaminide were also purchased from Sigma (St Louis, USA). Alcian blue was purchased from Edward Gurr (London, UK).
Alcian blue staining and degranulation index (DI) calculation of guinea pig peritoneal mast cells
Guinea pigs were first pre-sensitized with 2% CA (0.5 mL CA, ip, once every two days for ten days) and, ten days after the last pre-sensitization, peritoneal mast cells were collected. Mast cells were also collected from normal (non-sensitized) guinea pigs as a control. Both control and pre-sensitized guinea pigs were then injected ip with 10 mL calcium-free Tyrode's solution (4 °C) after the animals were killed by exsanguination under general anesthesia. After kneading the abdomen clockwise for 3–5 min, 5 mL of ascites fluid was collected and added to 30%/80% Percoll gradient centrifugation tubes and centrifuged at 2500 r/min for 15 min. All cells were washed twice in Tyrode's solution and then transferred to culture tubes after adjusting the cell density. The cells (3.7×105–5.2×105/mL) in each tube were treated with different concentrations of CA (0.2, 1.0, or 5.0 mmol/L) and stained with alcian blue after 30 min of incubation at 37 °C. Basophils were counted by microscopy, and the DI was calculated relative to negative control tubes.
DI=[(number of stained cells in control tube–number of stained cells in CA treated tube)/number of stained cells in control tube]×100%
Evaluation of RBL-2H3 cell exposure to CA
Light microscopic observation
RBL-2H3 cells in the exponential growth phase were incubated with different concentrations of CA (0.2, 1.0, or 5.0 mmol/L) and C48/80 (30 μg/mL), which was used as a positive control, for 30, 60, and 120 min at 37 °C. The reaction was terminated by incubating the tubes in an ice bath for 10 min. Cells were then stained with alcian blue for 10 min (a final concentration of 1.0×106 cells/mL was in each tube). A basophil count was performed, and the DI was calculated. Stained cell images were captured using a Motic AE30 EF-INV-III Inverted Fluorescence Microscope.
Transmission electron microscopic observation
For transmission electron microscopy, RBL-2H3 cells were treated with CA (0.2, 1.0, or 5.0 mmol/L) and C48/80 for 1 h. Then, the cells were fixed in glutaraldehyde–formaldehyde followed by osmium tetroxide, stained en bloc with uranyl acetate, embedded in Araldite and examined with a Hitachi (Tokyo, Japan) 600A electron microscope at 75 kV.
Fluorescent microscopic observation
According to the instructions provided with the annexin-V Fluos staining kit, RBL-2H3 cells, which had been treated with different concentrations of CA, were harvested, washed twice with cold PBS and resuspended in binding buffer containing FITC-conjugated annexin-V (5 μL). After 15 min in the dark, cells were mounted on a slide, examined under an Olympus fluorescent microscope and photographed using Kodak Elite II 100 slide film (Eastman Kodak, Rochester, NY).
Flow cytometric analysis
Cells were treated as discussed above. After 15 min in the dark with annexin-V, cells were analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA).
β-Hexosaminidase release
RBL-2H3 cells (2×105/well) in 24-well plates were washed with PIPES buffered solution. The cells were stimulated by different concentrations of CA at 37 °C under gentle rotation. The supernatants were collected and transferred to a 96-well plate. Subsequently, Triton X-100 solution (0.5%) was added to the cells to quantify the remaining enzyme activity. The extracts were transferred to another 96-well plate. The absorbance at 405 nm (OD) of each well was measured. The percentage of degranulation was calculated using the following formula:
% β-hexosaminidase release=ODsupernatant/(ODsupernatant+ODTriton X-100)×100.
Statistical analysis
All values are reported as means±standard deviation (SD). Statistical significance was analyzed using the Student's unpaired t-test. Values of P<0.05 were considered statistically significant.
Results
CA-induced degranulation in guinea pig peritoneal mast cells
As shown in Table 1, CA promoted the degranulation of guinea pig peritoneal mast cells in vitro but did not increase the peritoneal mast cell DI in CA-sensitized guinea pigs. These results suggest that CA may cause anaphylactoid reactions rather than anaphylactic reactions.
CA-induced degranulation in RBL-2H3 cells
Cell morphological changes
RBL-2H3 cells were adherent in monolayer, and a high degree of intracytoplasmic heterogeneity among particles could be observed with the inverted phase contrast microscope. RBL-2H3 cells appeared round with non-staining nuclei, and were evenly distributed within the light blue particulate after staining with alcian blue. After degranulation, RBL-2H3 cells showed deformation to vary degrees, including sparse intracellular particles, and even vacuolated cytoplasms and faded color. DI was calculated according to the formula shown above by counting the degranulated cells in the visual range after unifying the observation standards and adjusting cell concentrations; results are shown in Table 2. Our results demonstrate that CA can cause RBL-2H3 cell degranulation in a dose- and time-dependent manner.
Ultrastructural changes
Using transmission electron microscopy, we found clear nucleoli, cell membranes with many microvilli, cytoplasmic bubble-like particles of different sizes and some intracellular small dense particles in RBL-2H3 cells (culture) (Figure 1A). In cells treated with CA and C48/80, we observed chromatin condensation, dilatation of the endoplasmic reticulum, a decrease in intracellular particulates, sparse cytoplasm and a considerable increase in the number of vesicles and vacuole-like structures. Furthermore, typical characteristics of degranulation, including migration of granular vesicles toward the plasma membrane and integration combined with exocytosis, were observed (Figure 1B–1E). With increased CA concentration, increases in intracellular vacuole-like structures and degranulation effects were seen (Figure 1C–1E).
Fluorescent microscopy observation
As shown in Figure 2, in the low CA (0.2 mmol/L) group, only a few cells showed positive staining. As the CA concentration increased, the ratio of positive staining was markedly increased. The majority of cells in the high CA (5.0 mmol/L) group showed positive staining. Thus, CA can induce the concentration-dependent translocation of phosphatidylserine in RBL-2H3 cells.
Flow cytometric analysis
The positive staining ratio increased when the CA concentration was increased (Figure 3). This funding suggests that CA could induce the concentration-dependent translocation of phosphatidylserine in RBL-2H3 cells.
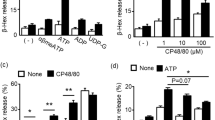
β-Hexosaminidase release
As shown in Table 3, there was no increase in β-hexosaminidase release in RBL-2H3 cells after incubation with a low concentration of CA (0.2 mmol/L) for 120 min. However, β-hexosaminidase release in RBL-2H3 cells significantly (P<0.01) increased after incubation with 1 mmol/L CA for 60 min and 5 mmol/L CA for 30 min. β-Hexosaminidase release was associated with the concentration of CA and the incubation time. This correlation provided indirect proof that CA can induce the degranulation of RBL-2H3 cells.
Discussion
Anaphylaxis and anaphylactoid reactions are clinically indistinguishable but differ in their pathogenic mechanisms. Anaphylaxis is a type I, or immediate, hypersensitivity reaction to an allergen. In this type of reaction, antigen-specific IgE binds to mast cells and basophils and is cross-linked by allergen, which triggers the release of preformed chemical mediators, such as histamine and tryptase, and then results in the multiorgan symptoms of anaphylaxis5, 6. Unlike anaphylactic reactions, anaphylactoid reactions are not dependent on drug-specific IgE and do not necessarily require previous exposure to an inciting substance. Many pathways can induce anaphylactoid reactions. Drugs can stimulate mast cells and basophils to release biologically active substances via physical or chemical mechanisms that are non-immune; alternatively, drugs can stimulate cells to release biologically active substances via complement activation (one of the mechanisms of the immune system) or other pathways7. Many drugs, such as radiocontrast media, non-steroidal anti-inflammatory drugs, analgesics, morphine, liposomes and micellar solvents, have been found to induce anaphylactoid reactions6. Because anaphylaxis and anaphylactoid reactions are clinically indistinguishable, reports of anaphylactoid reactions often include anaphylaxis in many studies6. Although anaphylactoid reactions induced by injections of TCMs have been reported rarely in the last few years, the degranulation of tissue mast cells and blood basophils can still be induced because TCMs have complex compositions and a large number of excipients. Anaphylactoid reactions may occur if the injections are delivered incorrectly, especially when too much intravenous drug is given within a short time period. It has been reported that anaphylactoid reactions can be induced in Beagle dogs and guinea pigs with polysorbate-80, which is widely used in TCM injections8.
Due to the absence of systemic studies involving anaphylactic or anaphylactoid reactions induced by CA, we decided to carry out a series of related experiments. Previous studies by our group showed that pure CA does not induce anaphylactic reactions in an active systemic anaphylactic assay and a passive cutaneous anaphylactic assay; our results are consistent with the outcome of a similar study by another group9, 10. However, our studies showed that the anaphylactoid reaction induced by intravenous injection of CA in guinea pigs is not related to sensitization and that the anaphylactoid symptoms (eg, trembling, salivation, tears and vomiting) could be observed during the first injection of CA in Beagle dogs (results not shown in this paper). In the present study, we find that CA can promote the degranulation of guinea pig peritoneal mast cells, but it does not increase the peritoneal mast cell DI in CA-sensitized guinea pigs. Our findings indicate that the effect of degranulation induced by CA is not related to pre-sensitization. This result serves as further evidence that CA can cause anaphylactoid reactions.
Anaphylaxis and anaphylactoid reactions share a common mechanism: the degranulation of mast cells or basophils. Therefore, the degranulation of mast cells and basophils is a key element in the study of anaphylaxis and anaphylactoid reactions. The continuous rat cell line, RBL-2H3, was cloned, using the limited dilution technique, from leukemic cells isolated from rats after treatment with the chemical carcinogen β-chloroethylamine11. RBL-2H3 cells have been extensively used for studying IgE–FcεRI interactions12, for studying signaling pathways of degranulation13 and for testing novel mast cell stabilizers14. An advantage of using the RBL-2H3 cell line is that a great number of monoclonal cells can be rapidly obtained by simple cell culture techniques; therefore, it is a useful tool for in vitro study.
Traditional methods of quantitatively measuring mast cell degranulation include calculating the DI (ie, direct observation and counting of cells stained with alcian blue) and observing the histamine release rate (ie, determination of histamine levels by ELISA or fluorospectrophotometry). However, ELISA sensitivity is too low to be useful in microanalysis because of the short half-life of histamine and instability of the fluorescent material. In recent years, the rates of β-hexosaminidase release and annexin-V positive cells have gradually become the preferred indices in degranulation research. β-Hexosaminidase is one of the many acid hydrolases present in mast cell granules and released from the cell membrane. Annexin-V is calcium-dependent and binds to phosphatidylserines exposed on the plasma membrane during apoptosis15. With mast cell activation, exogenous annexin-V binds to secretory granules on the cell surface in proportion to the degree of degranulation, as measured by β-hexosaminidase release16.
In this study, we demonstrated the direct induction of degranulation by CA in rat RBL-2H3 cells and investigated the cellular mechanisms of CA-induced anaphylactoid reactions. Our results showed that CA is able to dose dependently induce degranulation in RBL-2H3 cells. Furthermore, dose-dependent and time-dependent translocation of phosphatidylserine and release of β-hexosaminidase were found in RBL-2H3 cells following incubation with CA. This evidence demonstrates that CA induces degranulation in RBL-2H3 cells: this process may contribute to anaphylactoid reactions following intravenous injection of CA.
Because anaphylactoid reactions are dose- and concentration-dependent, and this study showed that CA is able to induce degranulation concentration dependently, caution should be exercised when using TCMs containing CA. It is recommended that drug labels should be followed strictly, doses should not be increased casually, and adverse events should be treated rapidly in order to avoid the occurrence of anaphylactoid allergy.
Author contribution
Dr Fang-hua HUANG, Prof Xin-yue ZHANG, and Prof Lu-yong ZHANG designed the study. Dr Fang-hua HUANG, Prof Xin-yue ZHANG, and Qin LI performed all of the experiments and analyses. Xiao-liang ZHENG and Ai-jun CHEN helped conduct part of the study. Prof Xin-yue ZHANG analyzed data. Dr Fang-hua HUANG, Prof Xin-yue ZHANG, and Dr Bin NI wrote the paper.
References
Lai YH, Chen HA, Yang WR . Allergy research of Chinese medicine injections should be strengthened. Tradit Chin Drug Res Clin Pharmacol 2002; 13: 324–6. Chinese.
Liang JQ, Zou YP, Den XC . Literature survey and analysis of adverse effects of Chinese medicine injections. Chin J Hosp Pharm 2003; 23: 40–2. Chinese.
Han QM, Jiang YF, Wu JH . Literature analysis of adverse effecto of Chinese medicine injections in our hospital. Xinjiang J Tradit Chin Med 2003; 21: 55–6. Chinese.
Huang FH . Safety analysis of traditional Chinese medicine injections with chlorogenic acid. China J Chin Mater Med 2008; 33: 2716–9. Chinese.
Berkes EA . Anaphylactic and anaphylactoid reactions to aspirin and other NSAIDs. Clin Rev Allergy Immunol 2003; 24: 137–47.
Leone R, Conforti A, Venegoni M . Drug-induced anaphylaxis: case/non-case study based on an italian pharmacovigilance database. Drug Saf 2005; 28: 547–56.
Chen F, Shi YQ, Qin HD, Liu ZP . Inquiry into the biomarkers of drug anaphylactoid reactions. Chem Life 2008; 28: 795–8. Chinese.
Sun L, Liu XM, Wang X, Qi WH, Shen LZ, Li B . Discover the reason of allergic reactions induced by Tween-80 in animals. J Toxicol 2007; 21: 322. Chinese.
Luo F, Bao X, Lin DS, Yang HR, Zhou S, Xu XP . Allergic study of chlorogenic acid to animal. West China J Pharm Sci 2009; 24: 181–3. Chinese
Li JF, Li Y, Chen Q, Cheng ZH, Tang S . Research of the Immuontoxity of Shuanghuangliana Injection. Tradit Chin Drug Res Clin Pharmacol 2008; 19: 172–4. Chinese.
Siraganian RP, McGivney A, Barsumian EL, Crews FT, Hirata F, Axelrod J . Variants of the rat basophilic leukemia cell line for the study of histamine release. Fed Proc 1982; 41: 30–4.
Ortega E, Schweitzer-Stenner R, Pecht I . Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast cells. EMBO J 1988; 7: 4101–9.
Funaba M, Ikeda T, Abe M . Degranulation in RBL-2H3 cells: regulation by calmodulin pathway. Cell Biol Int 2003; 27: 879–85.
Ikawati Z, Wahyuono S, Maeyama K . Screening of several Indonesian medicinal plants for their inhibitory effect on histamine release from RBL-2H3 cells. J Ethnopharmacol 2001; 75: 249–56.
Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 1995; 182: 1545–56.
Demo SD, Masuda E, Roosi AB, Throndset BT, Gerard AL, Chan EH, et al. Quantitative measurement of mast cell degranulation using a novel flow cytometric annexin-V binding assay. Cytometry 1999; 36: 340–8.
Acknowledgements
This work was supported by the Chinese Medicine Industry Research and Special Project (No 200707008) and by the Natural Science Foundation of Zhejiang Province (No Y206846).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Fh., Zhang, Xy., Zhang, Ly. et al. Mast cell degranulation induced by chlorogenic acid. Acta Pharmacol Sin 31, 849–854 (2010). https://doi.org/10.1038/aps.2010.63
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.63