Abstract
Aim:
To synthesize a novel polyamide SL-A92 and evaluate its bioactivity against drug resistance in Candida albicans.
Methods:
SL-A92 was synthesized using N-hydroxybenzotriazole (HOBT)/N,N'-dicyclohexylcarbodiimide (DCC) in solution phase. Its antifungal activities and effects on strain growth were tested using the micro-broth dilution method and growth curves, respectively. Induced drug resistance in the C. albicans collection strain SC5314 was obtained by incubation with fluconazole (12 μg/mL) for 21 passages. Meanwhile, incubations with SL-A92 plus fluconazole were also carried out in SC5314 strains, and the MIC80s were used to evaluate the inhibitory effects of SL-A92 on drug resistance during the induction process. Real time RT-PCR was performed to investigate the CDR1 and CDR2 mRNA levels in induced SC5314 strains.
Results:
SC5314 strain induced by the combination of fluconazole and SL-A92 (200 μg/mL) did not develop drug resistance. On day 24, the CDR1 and CDR2 mRNA levels in SC5314 strain co-treated with fluconazole and SL-A92 relative to fluconazole alone were 26% and 24%, respectively, and on day 30 the CDR1 and CDR2 mRNA levels were 43% and 31%, respectively.
Conclusion:
SL-A92 can block the development of drug resistance during the fluconazole induction process, which partially results from the down-regulation of CDR1 and CDR2.
Similar content being viewed by others
Introduction
Candida albicans (C albicans), the major opportunistic fungal pathogen of humans, causes various forms of candidiasis, ranging from superficial mucosal infections to life-threatening systemic disorders1, 2, 3. Extensive and repetitive use of antifungal azole derivatives such as fluconazole to treat refractory and recurrent candidosis infections has prompted C albicans to develop multiple mechanisms of multidrug resistance (MDR) in order to ensure its survival4, 5.
Many genes have been confirmed to participate in the development of MDR, including CDR1, CDR2, CaMDR1 and ERG116, 7, 8. Recently, we have demonstrated that RTA2 is also involved in calcineurin-mediated drug resistance in C albicans9, 10. Some transcription regulatory factors of these MDR genes have been identified, and many of them are zinc cluster proteins, such as Tac1p, Fcr1p and Upc2p11. Zinc cluster proteins form one of the largest families of transcriptional regulators in eukaryotes, displaying variable secondary structures and enormous functional diversity, and they can bind as homodimers to CGG triplets that are oriented in everted, inverted, or direct repeats11. These transcription factors, as well as the transcription processes, represent potential targets against MDR. Among the MDR genes regulated by zinc cluster transcription factors, the expression of CDR1 and CDR2 has received considerable attention, because their encoding ABC transporter proteins, Cdr1p and Cdr2p, could pump azoles out of cells to reduce azole accumulation as a self-defense mechanism12, 13, 14, 15. Our previous studies have also demonstrated that elevated CDR1 and CDR2 levels are associated with the progression of MDR in antifungal treatment16, 17.
Numerous attempts have been made to cope with clinical treatment failures resulting from drug resistance, such as developing novel antifungal compounds18, 19, 20, 21 and exploring combination therapies22, 23, 24, 25, 26, 27, 28, 29. Small molecule approaches for gene regulation could bypass the need for delivery strategies. A number of natural and synthetic DNA binding molecules have been explored for their ability to regulate gene expression in vitro and in vivo. Polyamides, containing N-methylimidazole (Im) and N-methylpyrrole (Py), represent one approach to inhibit protein-DNA interactions, and they comprise a class of programmable DNA-binding ligands that can bind to a broad repertoire of DNA sequences with affinities and specificities comparable to those of natural DNA-binding proteins. They are cell permeable, have no cytotoxicity, bind to chromatin and have been shown to inhibit a broad range of transcription factors resulting in the down-regulation of endogenous gene expression in cell culture30, 31. Sequence specificity is programmed by side-by-side pairings of the heterocyclic amino acids in the minor groove of DNA: Im/Py distinguishes GC from CG; Py/Py binds both AT and TA32, 33.
In this study, we designed and synthesized a DNA-binding polyamide SL-A92 to target the CCG within the binding site of the consensus zinc cluster proteins. We showed that SL-A92 had neither antifungal activities nor effects on strain growth and could not inhibit the drug resistance of the clinical isolate Y012. However, it could block the development of drug resistance in the SC5314 strain at a concentration of 200 μg/mL in fluconazole induction for 33 days. Furthermore, using real time RT-PCR, we investigated the regulation of the CDR1 and CDR2 mRNA levels by SL-A92 in the induced SC5314 strains.
Materials and methods
Synthesis of polyamide
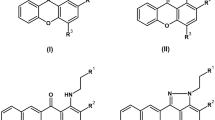
Synthesis of SL-A92 was carried out using a solution-phase approach according to the synthesis route (Figure 1). Thin-layer chromatography was performed on silica gel HSGF254 plates, and column chromatography was performed using silica gel (300-400 mesh). Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a BRUKER AVANCE II 300 NMR spectrometer. Compounds 1-3 were synthesized as described by Dervan34.
NO 2 PyCOOH (4)
NO2PyCOOMe (compound 1, 2 g, 7.37 mmol) was dissolved in methanol (50 mL), followed by the addition of 1 mol/L NaOH (30 mL). The reaction mixture was stirred at room temperature for 2 h. The methanol was removed, and the solution was washed with ethyl ether (2×50 mL). The pH of the aqueous layer was reduced to approximately 3 with 10% (v/v) HCl, and the mixture was extracted with ethyl acetate (3×50 mL). The combined ethyl acetate extracts were dried (sodium sulfate) and concentrated in vacuo to provide 1.2 g of NO2PyCOOH as a brown powder (91% yield). 1H NMR (DMSO-d6,300MHz): δ13.15(s, 1 H), 8.22(q, 1 H, J=1.5Hz), 7.25(d, 1 H, J=4.8Hz), 3.90(s, 3 H).
NO 2 ImImCOOEt (5)
NH2ImCOOEt (compound 3) was collected from 100 mg of NO2ImCOOEt as described by Dervan34 and then dissolved in ethyl acetate (30 mL). NO2ImCOOH (88.5 mg, 0.52 mmol) was added, followed by HOBT/DCC (80/120mg). The mixture was stirred for 4 h. DCU was removed by filtration. The filtrate was concentrated in vacuo and then purified by column chromatography using methanol and chloroform as an eluent (gradient eluate) to provide NO2ImImCOOEt as a light yellow powder (72.8 mg, 45% yield). 1H NMR (CDCl3-d1,600MHz): δ9.66(s, 1H), 7.78(s, 1H), 7.52(s, 1H), 4.43(d, 2H, J=7.2Hz), 4.18(s, 3H), 4.03(s, 3H), 1.44(t, 3H, J=7.2Hz).
NO 2 PyImImCOOEt (6)
Pd/C catalyst (10%, 10 mg) was added to a solution of NO2ImImOEt (100 mg, 0.31 mmol) in 15 mL of methanol, and the mixture was stirred under a slight positive pressure of H2 for 4 h. The catalyst was removed by filtration through Celite and washed with 50 mL of ethyl acetate. The filtration was concentrated in vacuo and then dissolved in 30 mL of DCM. NO2PyCOOH (54 mg, 0.32 mmol) was added, followed by the addition of HOBT/DCC (48/70 mg). The reaction solution was stirred for 4 h. SL-A92 was obtained as described in the synthesis of NO2ImImCOOEt to provide NO2PyImImCOOEt as a light yellow powder (84.1 mg, 61% yield). 1H NMR (DMSO-d6, 600 MHz): 8.17(d, 1 H, J=1.8 Hz), 7.70(d, 1 H, J=2.4 Hz), 7.67 (s, 1 H), 7.61 (s, 1 H), 4.30 (q, 2 H, J=7.2 Hz), 4.00 (s, 3 H), 3.97 (s, 3 H), 3.95 (s, 3 H), 1.32 (t, 3 H, J=7.2 Hz).
Drugs, strains and media
Fluconazole (2 mg/mL) was purchased from Pfizer Inc (New York, NY). SL-A92 was dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 10 mg/mL. The C. albicans collection strain SC5314 was kindly provided by William A FONZI (Department of Microbiology and Immunology, Georgetown University, Washington, DC). Clinically isolated C. albicans Y0109, 102 and Y012 strains were obtained from the Department of Dermatology, Changhai Hospital (Shanghai, China). The strains were cultivated at 30oC under constant shaking (200 r/min) in a liquid complete YPD medium consisting of 1% (w/v) yeast extract, 2% (w/v) peptone, and 2% (w/v) dextrose. RPMI 1640 medium was buffered to pH 7.0 with 3-(N-morpholino)-propane-sulfonic acid (MOPS) to a final concentration of 0.165 mol/L.
Antifungal susceptibility testing
The in vitro minimal inhibitory concentrations (MIC) of SL-A92 were determined using the micro-broth dilution method as defined by the National Committee for Clinical Laboratory Standards (NCCLS)35. SC5314, Y0109 and 102 were cultured in RPMI-1640 medium, with an inoculum concentration of 103 cells/mL. The final concentrations of SL-A92 ranged from 0.39 to 200 μg/mL. The microdilution plates inoculated with SL-A92 were incubated at 30oC. MIC endpoints for SC5314 were determined after incubation for 24 h. The drug MIC80 was defined as the first well with an approximate 80% reduction in growth compared to the growth of the drug-free well.
Growth curves
SC5314 in YPD medium was prepared at the starting inoculum of OD600=0.003. The concentrations of SL-A92 that were added were 5, 20 and 200 μg/mL and the concentration of fluconazole was 2 μg/mL. The growth was monitored by measuring the optical density at 600 nm (OD600) of the cultures throughout the subsequent 48 h at predetermined time points (0, 2, 4, 6, 8, 12, 16, 24, 36, and 48 h).
Antifungal drug resistance assay
A randomly selected colony of Y012 was inoculated in 1 mL of YPD medium and cultivated for 24 h. When the culture reached a density of 108 cells/mL, each aliquot of this culture containing 106 cells was then transferred to 1 mL×4 fresh YPD broth. SL-A92 was then added to three strains reaching the final concentrations of 5, 20 and 200 μg/mL, respectively. The fourth strain was supplemented with an equal amount of DMSO to broth and used as a blank control. Each day, 10 μL from each overnight culture was serially transferred into 1 mL of fresh medium, and the drug concentrations in the experiment were never reduced. At intervals of 4 days over the following 20 days of incubation, the susceptibilities of the induced Y012 strains were tested in each aliquot by the NCCLs microdilution method.
Induction of azole resistance and the identification
The incubation of SC5314 with SL-A92 at three concentrations was performed as with Y012. In addition, fluconazole 6 μL was added to each of the above strains to make up a final concentration of 12 μg/mL. Cultures with only DMSO or only the addition of fluconazole 6 μL were used as blank and positive controls, respectively. These five strains were induced as with Y012 for 33 days, and the susceptibilities were tested every 3 days. Furthermore, a 0.5 mL aliquot of the culture suspension, mixed with 0.5 mL of 80% glycerol, was frozen at -70oC for further study of the mechanism.
Real time RT-PCR
RNA isolation
Total RNA isolation was carried out using the Fungal RNA out kit (TIANDZ, Sichuan, China) as described in the manufacturer's manual. After quantifying the isolated total RNA, the yields were 20-50 μg per well, and genomic DNA was digested by treatment with DNase I (TaKaRa, Dalian, China). Purification of the RNA was carried out using the Column RNA clean kit (TIANDZ) to provide RNA for synthesis of the first strand cDNA.
Real time RT-PCR
The cDNA synthesis and PCR amplification were done as described previously36. RNA was standardized by quantifying the 18S gene as an endogenous control. All the primer sequences are listed in Table 1. Real time RT-PCR was performed independently in triplicate using the Chromo4 System (Bio-Rad). The gene expression level relative to the calibrator was expressed as 2−ΔΔCT36.
Results
Antifungal activity of SL-A92
The MIC80s against a laboratory collection strain of SC5314, and the clinically isolated drug-susceptible Y0109 and clinical isolated drug-resistant 102 strains were measured to evaluate the antifungal activity of SL-A92. As shown in Table S3, all the MIC80s of SL-A92 to the above three strains were >200 μg/mL, which indicates that there was no obvious antifungal activity of SL-A92.
Growth curve
Growth curves were plotted to evaluate the effects of SL-A92 on the growth of the strains. The OD600 was used to monitor the growth of the cultured strains. As illustrated in Figure 2, fluconazole at 2 μg/mL showed obvious inhibition on the growth of SC5314. However, the curves of the strains incubated with SL-A92 at 5, 20 and 200 μg/mL were in accordance with that of the only DMSO incubated strain within 48 h (for the OD600 data, Table S4). These curves indicate that SL-A92 did not affect the growth of the strains incubated at those concentrations.
Evaluation of azole resistance inhibition
The susceptibilities to fluconazole of the Y012 strains induced by SL-A92 at different concentrations were used. As shown in Table S5, the susceptibility did not change during any part of the induction process at each concentration, which suggests that SL-A92 had no significant inhibition on the drug resistance that had already developed.
Blocking the development of drug resistance during fluconazole induction
The susceptibilities of different induced SC5314 strains to fluconazole are shown in Table 2. On day 21, the positive control strain had already developed resistance, as indicated by an MIC80 > 64 μg/mL, and the resistance was stable in the following induction process. However, drug resistance did not appear in the strain with 200 μg/mL of SL-A92. The resistance of the strain with 20 μg/mL of SL-A92 was unstable, with MIC80s of 4 μg/mL and 2 μg/mL on day 21 and day 30, respectively. As 5 μg/mL of SL-A92 showed no effect on the drug resistance in S3, we speculate that the inhibition to drug resistance could affect the MIC80s when the concentration of SL-A92 in the co-incubation mixture reached 20 μg/mL. The results described here indicated that 200 μg/mL of SL-A92 could block the development of drug resistance during fluconazole induction.
Determination of the CDR1 and CDR2 mRNA levels in the induced SC5314
Real time RT-PCR was performed to determine the CDR1 and CDR2 mRNA levels of the induced strains. Strains were incubated with DMSO, fluconazole, and fluconazole combined with 200 μg/mL of SL-A92 for 24 days; the strains were coded as S1-24, S2-24 and S5-24, respectively. At the same concentrations, the strains incubated for 30 days were coded as S1-30, S2-30 and S5-30, respectively. The CDR1 and CDR2 mRNA levels of SC5314 were normalized to 1. As shown in Figure 3, both CDR1 and CDR2 in S1-24 and S1-30 were at almost the same levels as those of SC5314. However, the CDR1 level of S2-24 was up-regulated to 2.14-fold compared to that of SC5314, while the CDR2 level showed no significant change. The CDR1 and CDR2 levels of S5-24 were reduced to 26% and 24% compared to those of SC5314, respectively. Similarly, on day 30, the CDR1 level of S2-30 was up-regulated to 2.87-fold compared to that of SC5314, and the CDR2 level showed no significant change. The CDR1 and CDR2 levels of S5-30 were reduced to 43% and 31% of those of SC5314, respectively (for the relative fold change data, see supplementary Table 6). In other words, participation of SL-A92 in the induction process resulted in a decrease in CDR1 mRNA production as well as 88% and 85% the transcripts that were induced by fluconazole only in cultured SC5314 cells on days 24 and 30, respectively.
Discussion
Widespread and long-term use of azole derivatives to treat C albicans infection has promoted the development of MDR, which has become a general and severe problem in clinical therapy. In the present study, we designed and synthesized a cell-permeable, sequence-specific, DNA-binding polyamide SL-A92 to target the sequence CGG, which is the binding site of zinc cluster transcription factors, with the goal of interrupting some MDR gene expression and thus further inhibiting drug resistance.
Just as reported previously30, we also found that SL-A92 had no cytotoxicity, as revealed by its antifungal activity and effects on strain growth. Based on the theory31 of how polyamide exerts its function of gene regulation, a clinically isolated drug-resistant strain Y012 was incubated with SL-A92 for 20 days. Unfortunately, inhibition of drug resistance in induced Y012 strains was not found. On the one hand, this may be due to unknown resistance mechanisms in Y012; resistance to drugs can be conceived of as a gradually evolving process wherein various mechanisms may appear during the course of chemotherapy. On the other hand, we speculate that SL-A92 could not exhibit its function when some MDR genes have already been up-regulated; therefore, we produced a model in which SL-A92 exerts its function during the development of drug resistance. Interestingly, we succeeded in promoting SC5314 in developing drug resistance using fluconazole induction, and the concomitance of SL-A92 induction blocked this induced drug resistance successfully at 200 μg/mL. These results indicate that SL-A92 could block the development of drug resistance during fluconazole induction, but not the resistance that had already developed.
The reduced intracellular accumulation of drugs is a common mechanism of resistance, which is correlated with the increased expression of the genes CDR1 and CDR2 (members of the ABC efflux pump family) and of the gene CaMDR1 (a member of the MFS efflux pump family)37, 38, 39. Four kinds of CDR genes in C albicans have been cloned13, 40, 41, 42, but only over-expression of CDR1 and CDR2 resulted in azole resistance43. Tac1p is the major transcription factor needed for the regulation of CDR1 and CDR2, and is characterized by a highly conserved Zn(II)2Cys6 zinc finger motif formed by six cysteines that coordinate two zinc atoms within the DNA-binding domain11, 44, 45. Previous studies have demonstrated that the binding site of Tac1p lies in the drug-responsive element (DRE) and consists of a direct CGG repeat with four intervening nucleotides (CGGAA/TATCGG)44. In this study, we showed that fluconazole could up-regulate the level of CDR1 after induction for 33 days in vitro, but there was no up-regulation of CDR2. However, the participation of SL-A92 in the induction resulted in significant decreases of both CDR1 and CDR2 mRNA production, and both were down-regulated below normal levels.
In conclusion, we demonstrate here that SL-A92 can block the development of drug resistance during fluconazole induction, but cannot block the resistance that had already developed. This effect could be partially due to the down-regulation of CDR1 and CDR2, although the detailed mechanisms need further study. Future work is also needed to explore the molecular target of SL-A92 in C albicans and to optimize the cellular and nuclear uptake of such functionalized compounds. This would provide a new method of antidrug resistance in C. albicans. The results presented here are a promising proof of principle for further studies in this area. Our findings may open a new doorway for the development and design of new effective agents for the regulation of MDR genes.
Author contribution
Shao-long ZHU contributed new reagents, performed the research, and wrote the paper; Zhi-hui JIANG assisted the synthesis; Ping-hui GAO designed the bioactivity research; Yue QIU and Liang WANG analyzed the data; Yuan-ying JIANG and Da-zhi ZHANG revised the manuscript.
References
Ellis M, Richardson M, De Pauw B . Epidemiology. Hosp Med 2000; 61: 605–9.
Garber G . An overview of fungal infections. Drugs 2001; 61: 1–12.
Maertens J, Vrebos M, Boogaerts M . Assessing risk factors for systemic fungal infections. Eur J Cancer Care 2001; 10: 56–62.
Boken DJ, Swindells S, Rinaldi MG . Fluconazole-resistant Candida albicans. Clin Infect Dis 1993; 17: 1018–21.
White TC, Marr KA, Bowden RA . Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 1998; 11: 382–402.
Pemán J, Cantón E, Espinel-Ingroff A . Antifungal drug resistance mechanisms. Expert Rev Anti Infect Ther 2009; 7: 453–60.
Gulshan K, Moye-Rowley WS . Multidrug resistance in fungi. Eukaryot Cell 2007; 6: 1933–42.
Gbelska Y, Krijger JJ, Breunig KD . Evolution of gene families: the multidrug resistance transporter genes in five related yeast species. FEMS Yeast Res 2006; 6: 345–55.
Jia XM, Wang Y, Jia Y, Gao PH, Zhang LX, Jiang YY, et al. RTA2 is involved in calcineurin-mediated azole resistance and sphingoid long-chain base release in Candida albicans. Cell Mol Life Sci 2009; 66: 122–34.
Jia XM, Ma ZP, Jia Y, Gao PH, Zhang LX, Jiang YY, et al. RTA2, a novel gene involved in azole resistance in Candida albicans. Biochem Biophys Res Commun 2008; 373: 631–6.
MacPherson S, Larochelle M, Turcotte B . A fungal family of transcriptional regulators: the Zinc cluster proteins. Microbiol Mol Bio Rev 2006; 70: 583–604.
Krishnamurthy S, Gupta V, Snehlata P, Prasad R . Characterization of human steroid hormone transport mediated by Cdr1p, a multidrug transporter of Candida albicans, belonging to the ATP binding cassette superfamily. FEMS Microbiol Lett 1998; 158: 69–74.
Sanglard D, Ischer F, Monod M, Bille J . Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 1997; 143: 405–16.
Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, Monk BC, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev 2009; 22: 291–321.
Gyorgy S, Karl K . Fungal ATP-binding cassette (ABC) transporters in drug resistance & detoxification. Curr Drug Targets 2006; 7: 471–81.
Shen H, An MM, Wang DJ, Xu Z, Zhang JD, Jiang YY, et al. Fcr1p inhibits development of fluconazole resistance in Candida albicans by abolishing CDR1 induction. Biol Pharm Bull 2007; 30: 68–73.
Gao PH, Cao YB, Jia XM, Cao YY, Quan H, Jiang YY, et al. Drug susceptibilities of yeast cells are affected when expressing mutant Candida albicans drug resistance protein. Int J Antimicrob Agents 2006; 28: 69–74.
Gao PH, Cao YB, Xu Z, Zhang JD, Zhang WN, Jiang YY, et al. In vitro antifungal activity of ZJ-522, a new triazole restructured from fluconazole and butenafine, against clinically important fungi in comparison with fluconazole and butenafine. Biol Pharm Bull 2005; 28: 1414–7.
Zhao JX, Cao YY, Quan H, Liu CM, He QQ, Jiang YY, et al. In vitro and in vivo activities of HQQ-3, a new triazole antifungal agent. Biol Pharm Bull 2006; 29: 2031–4.
Zhang JD, Cao YB, Xu Z, Sun HH, An MM, Jiang YY, et al. In vitro and in vivo antifungal activities of the eight steroid saponins from Tribulus terrestris L with potent activity against fluconazole-resistant fungal pathogens. Biol Pharm Bull 2005; 28: 2211–5.
Zhang JD, Xu Z, Cao YB, Chen HS, Yan L, Jiang YY, et al. Antifungal activities and action mechanisms of compounds from Tribulus terrestris L. J Ethnopharmacol 2006; 103: 76–84.
Lewis RE, Kontoyiannis DP . Rationale for combination antifungal therapy. Pharmacotherapy 2001; 21: 149S–164S.
Han Y, Lee JH . Berberine synergy with amphotericin B against disseminated candidiasis in mice. Biol Pharm Bull 2005; 28: 541–4.
Quan H, Cao YY, Xu Z, Zhao JX, Gao PH, Jiang YY, et al. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob Agents Chemother 2006; 50: 1096–9.
Huang S, Cao YY, Dai BD, Sun XR, Zhu ZY, Jiang YY, et al. In vitro synergism of fluconazole and baicalein against clinical isolates of Candida albicans resistant to fluconazole. Biol Pharm Bull 2008; 31: 2234–6.
Barchiesi F, Falconi Di Francesco L, Scalise G . In vitro activities of terbinafine in combination with fluconazole and itraconazole against isolates of Candida albicans with reduced susceptibility to azoles. Antimicrob Agents Chemother 1997; 41: 1812–4.
D'Auria FD, Tecca M, Strippoli R, Simonetti N . In vitro activity of propyl gallate-azole drug combination against fluconazole- and itraconazole-resistant Candida albicans strains. Lett Appl Microbiol 2001; 32: 220–3.
Sasaki E, Maesaki S, Miyazaki Y, Yanagihara K, Tomono K, Kohno S, et al. Synergistic effect of ofloxacin and fluconazole against azole-resistant Candida albicans. J Infect Chemother 2000; 6: 151–4.
Gil-Lamaignere C, Müller FM . Differential effects of the combination of caspofungin and terbinafine against Candida albicans, Candida dubliniensis and Candida kefyr. Int J Antimicrob Agents 2004; 23: 520–3.
Hsu CF, Phillips JW, Trauger JW, Farkas ME, Belitsky JM, Dervan PB, et al. Completion of a programmable DNA-binding small molecule library. Tetrahedron 2007; 63: 6146–51.
Dervan PB, Edelson BS . Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Curr Opin Struct Biol 2003; 13: 284–99.
Kielkopf CL, Baird EE, Dervan PD, Rees DC . Structural basis for G.C recognition in the DNA minor groove. Nat Struct Biol 1998; 5: 104–9.
White S, Szewczyk JW, Turner JM, Baird EE, Dervan PB . Recognition of the four Watson-Crick base pairs in the DNA minor groove by synthetic ligands. Nature 1998; 391: 468–71.
Baird EE, Dervan PB . Solid phase synthesis of polyamides containing imidazole and pyrrole amino acids. J Am Chem Soc 1996; 118: 6141–6
Ghannoum MA, Ibrahim AS, Fu Y, Shafiq MC, Edwards JE Jr, Criddle RS . Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J Clin Microbiol 1992; 30: 2881–6.
Wang Y, Cao YY, Jia XM, Cao YB, Gao PH, Jiang YY, et al. Cap1p is involved in multiple pathways of oxidative stress response in Candida albicans. Free Radic Biol Med 2006; 40: 1201–9.
Sanglard D, Ischer F, Monod M, Bille J . Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother 1996; 40: 2300–5.
Sanglard D, Kuchler K, Ischer F, Pagani JL, Monod M, Bille J . Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother 1995; 39: 2378–86.
White TC . Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother 1997; 41: 1482–7.
Prasad R, De Wergifosse P, Goffeau A, Balzi E . Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet 1995; 27: 320–9.
Balan I, Alarco AM, Raymond M . The Candida albicans CDR3 gene codes for an opaque-phase ABC transporter. J Bacteriol 1997; 179: 7210–8.
Franz R, Michel S, Morschhauser J . A fourth gene from the Candida albicans CDR family of ABC transporters. Gene 1998; 220: 91–8.
St Georgiev V . Membrane transporters and antifungal drug resistance. Curr Drug Targets 2000; 1: 261–84.
Coste AT, Karababa M, Ischer F, Bille J, Sanglard D . TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell 2004; 3: 1639–52.
Talibi D, Raymond M . Isolation of a putative Candida albicans transcriptional regulator involved in pleiotropic drug resistance by functional complementation of a pdr1 pdr3 mutation in Saccharomyces cerevisiae. J Bacteriol 1999; 181: 231–40.
Acknowledgements
This work was supported by grants from the National Natural Science Fundation of China (No 30672533; 30772644), Shanghai Pujiang Program (No 05PJ14003) and the “11th Five Year Plan” for the Scientific Program of PLA (06H013). We thank William A FONZI for kindly providing the C albicans collection strain SC5314.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary information accompanies this paper on the Acta Pharmacologica Sinica website .
Supplementary information
Supplementary materials
Here are results of SL-A92 antifungal activities, antidrug resistance activities and original data of this manuscript. (DOC 58 kb)
Rights and permissions
About this article
Cite this article
Zhu, Sl., Jiang, Zh., Gao, Ph. et al. A novel polyamide SL-A92 as a potential fungal resistance blocker: synthesis and bioactivities in Candida albicans. Acta Pharmacol Sin 31, 855–860 (2010). https://doi.org/10.1038/aps.2010.59
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.59
Keywords
This article is cited by
-
Late Cretaceous granitic magmatism and Sn mineralization in the giant Yinyan porphyry tin deposit, South China: constraints from zircon and cassiterite U–Pb and molybdenite Re–Os geochronology
Mineralium Deposita (2021)
-
Early Silurian Wuchuan–Sihui–Shaoguan exhalative sedimentary pyrite belt, South China: constraints from zircon dating for K-bentonite of the giant Dajiangping deposit
Acta Geochimica (2021)