Abstract
Aim:
To examine whether two naturally occurring sesquiterpenoids (ST1 and ST2) with anti-proliferative activity in prostate cancer cells inhibit androgen receptor (AR) signaling.
Methods:
Human prostate cancer cell lines LNCaP and PC3 were used. The expression of AR, AR translocation into the nucleus, and expression levels of AR coactivators ARA70 and steroid receptor coactivator-1 (SRC-1) in LNCaP cells were examined using real-time PCR and Western blot. Changes in prostate-specific antigen (PSA) protein levels, PSA promoter activity, and androgen response element (ARE)-mediated reporter gene activity were examined using enzyme-linked immunoabsorbent assay (ELISA) and transient transfection assays. Co-immunoprecipitation was performed to analyze the interaction between AR and the AR coactivators in ST1- and ST2-treated cells.
Results:
In LNCaP cells, ST1 and ST2 (40 μmol/L) led to a significant decrease in the expression of AR as well as a reduction of AR translocation into the nucleus, but had no effect on AR protein translation. ST1 and ST2 treatment also resulted in a significant decrease in the level of PSA protein secreted into the medium and was able to suppress PSA promoter-dependent and ARE-dependent luciferase activity. Furthermore, decreased expression of ARA70 and SRC-1 was observed when LNCaP cells were exposed to ST1 and ST2, which interfered with their ability to interact with AR.
Conclusion:
The observations suggest that suppression of AR transactivation by ST1 and ST2 may be mediated, in part, by inhibiting AR nuclear translocation and/or interfering with the interaction between AR and its coactivators ARA70 and SRC-1. Therefore, sesquiterpenoids could be developed as novel therapeutic agents for treating prostate cancer.
Similar content being viewed by others
Introduction
The biological roles of androgens in the prostate are mediated through the androgen receptor (AR), which is a ligand-activated transcription factor in the nuclear receptor superfamily that is required for normal development and maintenance of male sexual behavior1. Additionally, there is strong evidence demonstrating that androgen/AR signaling is involved in the development and progression of prostate cancer (PCa). The importance of AR in PCa is supported by numerous observations showing that AR has been detected and remains active during all stages of PCa, including in most hormone refractory prostate cancer (HRPC), suggesting that AR is improperly activated in the absence of or at post-castration levels of androgens2, 3, 4. Therefore, inhibition or reduction of aberrant AR activity is a major therapeutic goal for the management of metastatic disease5.
Myrrh is a resinous substance obtained from Commiphora trees, which is believed to act as an anti-tumor agent and is also capable of relieving pain. It has been combined with gum resins in the anti-tumor prescription drug Xihuang wan (or Xihuang pill) for the treatment of cancer in China6, 7. Sesquiterpenoids, nonsteroidal compounds found in myrrh, possess diverse biological functions, including antibacterial, anesthetic, and anti-hyperglycemic activity8, 9, 10. Recent studies have shown that sesquiterpenoids may be anti-tumorigenic11, 12, 13, but the molecular mode of action remains unknown. We have previously reported that two sesquiterpenoids isolated from myrrh, 1(10)E,2R,4R-2-methoxy-8,12-epoxygermacra-1(10),7,11-trien-6-one (ST1) and 2-methoxy-5-acetoxy-furanogermacr-1(10)-en-6-one (ST2), inhibited the proliferation of LNCaP cells14, 15. In this study, we examined whether the anti-tumorigenic function of ST1 and ST2 occurred targeting the AR. Our results showed that ST1 and ST2 blocked AR expression and transcriptional activity and that this inhibition interfered with the interaction of AR and its coactivators ARA70 and SRC-1.
Materials and methods
Cell culture and treatments
Human prostate cancer cell lines LNCaP (obtained from the American Type Culture Collection, Rockville, MD, USA) and PC3 (purchased from the Cell Bank of Chinese Academy of Sciences, Shanghai) were seeded in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; JRH, St Louis, MO, USA) and were kept in 5% carbon dioxide at 37 °C until they reached approximately 50% to 70% confluency. Cells were maintained in serum-free RPMI-1640 medium for 24 h to deplete endogenous steroid hormones and then treated with sesquiterpenoids (40 μmol/L) dissolved in RPMI-1640 medium containing 1% charcoal stripped FBS with or without 1 nmol/L synthetic androgen mibolerone (Mib). The sesquiterpenoids were dissolved in dimethyl sulfoxide (DMSO), which was also used as the control vehicle. The control group received the same volume of DMSO.
Western blot analysis
LNCaP cells were grown in 75-mL culture flasks using the same treatment described above. To test whether ST1 and ST2 affected AR protein translation, cells were pretreated simultaneously with cycloheximide (CHX, 20 μg/mL) and sesquiterpenoids (40 μmol/L) for 24 h. Removal of the media was followed by a brief rinse with cold PBS, and whole cell extracts were prepared as described previously16. Freshly prepared protease inhibitors [0.5 mmol/L phenylmethanesulfonyl fluoride (PMSF), 50 μg/mL aprotinin, 1 mmol/L sodium orthovanada te, 10 mmol/L sodium fluoride, and 10 mmol/L β-glycerolphosphate] were also added. The Bradford protein assay (Bio-Rad, Hercules, CA, USA) was employed for quantifying the protein content. Proteins were loaded onto an SDS polyacrylamide gel (8%) and electrotransferred onto a nitrocellulose membrane (PALL, Port Washington, NY, USA). The blots were blocked with 5% non-fat milk in TBST buffer (20 mmol/L Tris-HCl, 137 mmol/L NaCl, and 0.1% Tween 20, pH 8.0) prior to incubation with specific antibodies to AR (BD, Franklin Lakes NJ, USA), β-actin (Santa Cruz, CA, USA), ARA70 (Santa Cruz, CA, USA), and SRC-1 (Thermo, Rockford, IL, USA) for 1 h at room temperature. After three times of washing with TBST buffer, the membranes were incubated with an anti-rabbit or anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (Santa Cruz, CA, USA) at room temperature and visualized using enhanced chemiluminescence substrate (ECL, Amersham Corporation, Piscataway, NJ, USA).
Nuclear extracts
After treatments with sesquiterpenoids for 24 h, the LNCaP cells were pelleted, and the nuclear extracts, with or without STs treatment, were prepared as described previously16. The protein concentrations of the nuclear extracts were determined using the Bradford protein assay and stored at -80 °C in small aliquots.
Quantitative PCR
After exposure to ST1 and ST2 for 24 h in 6-well plates, LNCaP cells were collected, and the total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA). Changes in the mRNA levels of AR following ST1 and ST2 treatment were quantified using real-time RT-PCR on the ABI 7000 Sequence Detection System (Applied BioSystems, Carlsbad, CA, USA). The AR transcript was detected from M-MLV reverse transcriptase-amplified cDNA, with one aliquot designated to receive no enzyme. Quantitative PCR (TaqMan PCR) was performed using Premix Ex TaqTM reagent, according to the manufacturer's recommended protocols (Takara Biotechnology, Dalian, China). For each 25-μL TaqMan PCR reaction, synthesized cDNA, corresponding to 100 ng total RNA as a template, a final concentration of 400 nmol/L primers, 120 nmol/L of probe, 2×TaqMan PCR Mix and PCR-grade water were mixed together. Sequence-specific primers for AR were 5′-AAGGCTATGAATGTCAGCCCA-3′ (sense) and 5′-CATTGAGGCTAGAGAGCAAGGC-3′ (antisense). Probes contained the fluorescence reporter FAM at the 5′-end and TAMRA at the 3′-end, FAM5′-TGTGTGCTGGACACGACAACAACC-3′TAMRA. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included as an internal control and ran in the same PCR reaction. The probe and primer combinations were as follows: FAM5′-AACAGCGACACCCACTCCTCCACC-3′TAMRA, 5′-CCAGGTGGTCTCCTCTGACTT-3′ (sense) and 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ (antisense). All of the primers and probes were synthesized by Takara Biotech (Dalian, China). All of the PCR assays were performed in triplicate. The quality of each reaction was confirmed by comparing the triplicate RT versus the no-enzyme control. The amount of each target gene relative to GAPDH for each sample was analyzed using the 2-ΔΔCT method17. The values were indicated as the percentage of untreated control and set to 100%.
Transient transfection and reporter gene activity assays
The PC-3 and LNCaP cells were seeded in 24-well plates and grown under the conditions described above. A plasmid containing the AR promoter (-1380/+577) (AR 2 kb promoter, 0.8 μg/well), pGL3 basic vector with 6 kb of the PSA promoter (pGL3-PSA promoter, 0.8 μg/well), or pGL3-SV40 with three copies of the androgen response element (ARE) of the hk2 gene (hk2-3ARE, 0.8 μg/well) were transfected into LNCaP cells using LipofectamineTM 2000 (Invitrogen, Carlsbad, CA, USA). For transfection of DNA into PC3 cells, the human AR expression vector pSG5-AR (hAR, 0.2 μg/well) was included for cotransfection with the plasmids described above. The parental vectors pGL3 basic (0.8 μg/well) and pGL3-SV40 (0.8 μg/well) were used as controls. The phRL-TK vector (0.1 μg/well, Renilla luciferase, Promega, Madison, WI, USA) served as an internal control to normalize the transfection efficiency. After 24 h post-transfection, cells were either treated with sesquiterpenoids (40 μmol/L) or remained untreated in the presence or absence of 1 nmol/L Mib for an additional 24 h in medium containing 1% charcoal stripped serum. The cell extracts were prepared and used for luciferase assays (Dual-Luciferase Reporter Assay System, Promega, Madison, WI, USA). At least three independent transfection experiments were performed. Statistical analysis was done using two-tailed Student's t-test. P<0.05 was accepted as the level of significance.
Immunofluorescence staining
The LNCaP cells were cultured on slides. After sesquiterpenoid (40 μmol/L) treatment, LNCaP cells were fixed with 4% paraformaldehyde for 20 min. After washing with PBS, the cells were treated with 0.3% Triton X-100 in PBS for 15 min at room temperature to increase cellular permeability. Fixed cells were blocked with 10% normal goat serum in PBS at room temperature for 60 min, and then anti-AR antibody was applied at a dilution of 1:2 and incubated at 4 °C in a moist chamber overnight. The slides were then incubated with the FITC-conjugated goat anti-rabbit IgG (Zhongshan, China) for 60 min and examined under a fluorescence microscope (IX-71, Olympus, Tokyo, Japan). Negative control cells were incubated with preimmune rabbit serum instead of primary antibodies. The assays were repeated three times.
Measurement of secreted PSA protein
The LNCaP cells were cultured as described above and exposed to sesquiterpenoids in the absence or presence of Mib. After 24 h of incubation, the depleted media was harvested, and the levels of PSA in the depleted media were quantified with ELISA (Alpha Diagnostic, San Antonio, TX, USA). The cell density was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2Htetrazolium bromide (MTT; Sigma, St Louis, MO, USA) assay. The levels of PSA protein were normalized based on the cell density measurements.
Co-immunoprecipitation
The LNCaP cells were plated in 75-mL culture dishes and treated with sesquiterpenoids in the absence or presence Mib for 24 h. Whole cell lysates were prepared as described previously16 and precleared with anti-mouse IgG and protein A-agarose (Santa Cruz, CA, USA). Protein aliquots of 500 μg were incubated with 2 μg of antibody directed against AR (BD, Franklin Lakes, NJ, USA) in binding buffer (20 mmol/L HEPES, pH 7.9, 20% glycerol, 150 mmol/L KCl, 0.2 mmol/L EDTA, 0.5 mmol/L DTT, 0.5 mmol/L PMSF, 50 mg/mL aprotinin, 1 mmol/L sodium orthovanadate, 10 mmol/L sodium fluoride, and 10 mmol/L β-glycerolphosphate) at 4 °C overnight. The protein A-agarose beads were added and incubated for 6 h at 4 °C. The immunoprecipitates were washed four times with buffer containing 50 mmol/L Tris-HCl, pH 7.5, 0.5% IGEPAL CA-630, 150 mmol/L NaCl, 0.2 mmol/L EDTA, 0.5 mmol/L PMSF, 50 mg/mL aprotinin, 1 mmol/L sodium orthovanadate, 10 mmol/L sodium fluoride, and 10 mmol/L β-glycerolphosphate. Immunocomplexes were recovered by heating at 75 °C for 10 min in SDS sample buffer and analyzed by Western blot.
Results
ST1 and ST2 inhibit the expression of AR in LNCaP cells
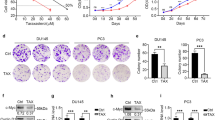
We previously reported that ST1 and ST2 inhibited LNCaP cell proliferation by causing cell cycle arrest in the G1 phase15. Because downregulation of AR resulted in significant suppression of prostate tumor cell growth18, 19, we used Western blot analysis to determine whether the inhibition of cell growth by STs was due to a reduction in AR expression in LNCaP cells. As shown in Figure 1A, treatment of LNCaP cells with Mib led to a significant increase in the expression of AR, while Mib-mediated stimulation of AR protein levels was decreased by ST1 and ST2. Activated AR typically translocates to the nucleus to regulate transcription of target gene expression. The effect of ST1 and ST2 on AR nuclear translocation was further analyzed by Western blot analysis of the nuclear extracts. As shown in Figure 1A, Mib treatment also increased the level of AR protein expression in the nucleus. This increase was drastically reduced by treating LNCaP cells with ST1 and ST2. These results indicate that the inhibition of androgen-stimulated AR nuclear translocation by ST1 and ST2 treatments might attribute to the suppression of AR protein expression. In addition, immunocytochemistry was performed to determine the change in AR protein levels in LNCaP cells. Similarly, in the absence of Mib, AR protein levels were reduced (Figure 1B), whereas Mib treatment caused a significant increase in the expression of AR in these cells. Furthermore, most of the AR was located in the nucleus. Consistent with the Western blot results, the AR protein level was greatly reduced in cells exposed to ST1 and ST2.
Effects of ST1 and ST2 on AR expression in LNCaP cells. (A) Western blot analysis of AR protein expression in whole cell lysates (WE) and nuclear extracts (NE) from LNCaP cells treated with or without STs was performed. Beta-actin and H1 bands represent protein loading and transferring efficiency controls. Histograms represent the densitometry analysis of Western blot. (B) Immunofluorescent staining of AR. (C) Changes in AR mRNA expression were determined by quantitative PCR. RNA from ST-treated and control cells were subjected to real-time PCR analysis using primers specific for AR or GAPDH, respectively. The data are shown as the mean±SD of three independent experiments, each performed in triplicate. bP<0.05 vs Mib treatment. (D) Effect of ST1 and ST2 on AR promoter activity. Cell extracts from transfections were used for dual luciferase activity assays. The resulting luciferase activity was normalized to the activity of phRL-TK to show the equal transfection efficiency. The normalized relative luciferase activities (mean±SD) of at least three independent experiments are shown. bP<0.05 vs Mib treatment.
To determine whether STs affect AR expression at the transcriptional level, we performed real-time PCR to monitor changes in the AR mRNA levels. Figure 1C showed that androgen-induced expression of the AR transcript was greatly reduced in cells exposed to STs compared with those treated with Mib alone. The inhibitory effect of STs on AR gene expression was further confirmed in LNCaP cells transfected with an AR promoter-luciferase construct. As shown in Figure 1D, increased AR promoter reporter activity was evident in response to Mib, and the luciferase activity was significantly inhibited by ST1 and ST2 in the presence of Mib, which is consistent with the data in Figures 1A, 1B, and 1C.
We next examined the effect of STs on AR protein synthesis using CHX, an inhibitor of protein synthesis. As shown in Figure 2, AR protein levels were decreased in the presence of CHX, whereas treatment with both CHX and STs caused no detectable changes in the AR protein expression, suggesting that STs may not regulate translation of the AR protein. These results indicate that the suppression of AR protein levels by ST1 and ST2 is mediated by a reduction of the AR mRNA transcripts in the presence of androgen.
Effects of ST1 and ST2 on AR protein synthesis in LNCaP cells. AR in LNCaP cells exposed to ST1 and ST2 in the presence or absence of CHX was analyzed using Western blot. Beta-actin is the protein loading and transferring efficiency control. Histograms show the densitometry analysis of Western blot.
ST1 and ST2 repress AR transcriptional activity
PSA and hk2 are androgen-inducible genes that contain AREs, which are AR-binding regions. The expression of the PSA and hk2 genes are highly dependent upon the regulation of androgens through AR20, 21. To clarify whether the ST-mediated reduction in cellular AR protein levels was accompanied a decrease in the transcriptional activity of the AR, the secreted PSA protein level was examined in ST-treated LNCaP cells. As shown in Figure 3, the secreted PSA level was enhanced in the presence of Mib compared to the untreated control, while exposure of LNCaP cells to ST1 and ST2 for 24 h decreased the PSA protein levels in the presence of androgens. The inhibition of ST1 and ST2 on AR transactivity was further investigated using co-transfection experiments. A construct containing the PSA promoter linked to a luciferase gene was transfected into LNCaP cells to examine the AR transactivity following ST treatment. As shown in Figure 4A, Mib stimulated the induction of the reporter gene, which was detected by examining the activity of luciferase, while ST1 and ST2 suppressed the androgen induction of the PSA promoter. We next transfected the PSA promoter-luciferase reporter, with an AR expression vector, into PC-3 cells lacking AR protein expression to confirm the ST-mediated inhibitory effect on AR transcriptional function. As shown in Figure 4B, the PSA promoter caused a strong androgenic induction of luciferase activity in Mib-treated cells. However, ST1 and ST2 treatments abolished the androgenic-mediated induction of the PSA promoter. Because ARE is necessary for AR-mediated gene transactivation, we used the luciferase reporter linked to three repeats of ARE from the hk2 gene to test whether specific DNA sequence dependent AR transcriptional activity could be affected by ST treatments. The luciferase activity of hk2-3ARE was reduced by ST1 and ST2 in the presence of Mib (Figure 4B). Additionally, the results in Figure 4D further established the efficacy of ST1 and ST2 by specifically blocking AR actions in PC3 cells. Thus, ST1 and ST2 treatments significantly suppressed AR expression and the androgen-stimulated AR transcriptional activity.
Effects of ST1 and ST2 on the AR transcriptional activity in LNCaP cells (A) Luciferase activity in LNCaP cells transfected with the pGL3-PSA 6 kb promoter luciferase reporter following treatment with STs for 24 h. (B) Luciferase activity in PC3 cells co-transfected with the pGL3-PSA 6 kb promoter reporter and a human AR expression construct following exposure to STs as indicated for 24 h. (C) Luciferase activity in LNCaP cells transfected with the pGL3-SV40-hk2-3ARE reporter following treatment with STs for 24 h. (D) Luciferase activity in PC3 cells co-transfected with the pGL3-SV40-hk2-3ARE reporter and a human AR expression construct following treatment with STs as indicated for 24 h. bP<0.05, cP<0.01 vs Mib treatment. The parental vectors pGL3 basic vector and pGL3-SV40 were included as controls. The phRL-TK, co-transfected in each transfection, was the internal control for normalization. The normalized relative luciferase activity (mean±SD) of at least three independent experiments is shown.
ST1 and ST2 weaken the interaction between AR and its coactivators SRC-1 and ARA70 in LNCaP cells
It is well documented that the AR intrinsic ligand-dependent activity is potentiated through its interaction with coactivators, including SRC-1 and ARA7022. Because ST1 and ST2 repressed the AR transcriptional activity and subsequently led to a reduction in the expression of PSA protein and promoter activity, it is likely that ST1 and ST2 suppressed AR transactivation by interrupting the function of AR coactivators. Alterations in the expression of ARA70 and SRC-1 were analyzed in response to ST1 and ST2 by Western blot. As shown in Figure 5, Mib treatment resulted in elevated expression of both ARA70 and SRC-1; however, protein abundance of these two coactivators was greatly reduced in LNCaP cells treated with ST1 and ST2. Co-immunoprecipitation was performed to further analyze the interaction between AR and its coactivators. Proteins potentially associated with AR were first precipitated with anti-AR antibody and subsequently detected using AR antibody, ARA70 antibody or SRC-1 antibody. As indicated in Figure 6, endogenous ARA70 and SRC-1 bands were detected in anti-AR-precipitated complexes from cells treated with Mib (line 2), and very weak signals were observed in ST1- and ST2-treated cells (lines 3 and 4). No detectable proteins were shown in immunocomplexes precipitated with normal IgG. Together, these data indicate that the ST1- and ST2-mediated reduction in the levels of AR coactivators ARA70 and SRC-1 also plays a role in the ST-induced suppression of AR transactivity, mainly through their inhibition of expressions of these two coactivators, and leading to interference with the interactions with the AR.
Effects of ST1 and ST2 on the interactions between AR and AR coactivators SRC-1 and ARA70. The association of AR and AR coactivators SRC-1 and ARA70 was analyzed using co-immunoprecipitation. Normal IgG was used as the negative control. Input represents 10% of the whole cell extracts used in the above experiments.
Discussion
The present work is an initial study aimed at identifying novel, natural nonsteroidal modulators targeting androgen/AR signaling from traditional oriental medicines. Based on previous studies using petroleum extract from myrrh, we discovered two sesquiterpenoids, ST1 and ST2, that inhibited prostate cancer cell proliferation. In this study, we demonstrated that the sesquiterpenoids suppressed AR promoter transcription, mRNA and protein expression levels and decreased androgen-stimulated PSA promoter activity and protein secretion. These observations indicated that ST-mediated suppression of AR function was at least partly due to a reduction of AR expression in the nucleus.
To regulate the transcription of target genes, AR must recruit a series of coactivator proteins that generally do not directly bind to DNA. These proteins are recruited to gene-promoter regions through protein–protein interactions with AR, and this interaction occurs usually in a ligand-dependent manner. Therefore, a decrease in the expression levels of AR coactivators or the interruption of their interaction with AR in prostate cancer cells could contribute to AR signaling inhibition. The first identified member of the coactivator family that regulated steroid receptor action was SRC-123, which is functional in many different tissue types, enhances transcriptional activity of the AR in a ligand-dependent manner22, and is involved in the negative regulation of AR activity by sesquiterpenoids. Sesquiterpenoids significantly decreased the expression of SRC-1 and interfered with the interaction between the AR and SRC-1 in the presence of androgens. In addition, ARA70 has been reported to be a relatively AR-specific coactivator22; however, other studies contradict this idea. The expression of ARA70 is increased in high-grade prostate cancer tissues as well as in hormone-refractory LNCaP xenografts and other prostate cancer cell lines. High levels of ARA70 may induce agonist activity of anti-androgens in LNCaP and other hormone-refractory prostate cancer cells24, 25. The molecular mechanisms by which ARA70 enhances AR transactivation involve increasing AR expression, protein stability, and nuclear translocation. In this study, when cells were exposed to the sesquiterpenoids, ARA70 protein expression was inhibited, and the interaction between AR and ARA70 was also interfered. Further studies are necessary to test if the sesquiterpenoids, by inhibiting ARA70, can enhance the anti-androgen treatment of the advanced stages of prostate cancer.
As sesquiterpenoids can decrease ARA70 and SRC-1 levels and interfere with the interaction between AR and its coactivators, our data support the idea that the inhibition of AR transcriptional activity by these two compounds occurred in an androgen-dependent manner. However, it remains to be clarified whether sesquiterpenoids can block androgen binding to AR and inhibit AR translocation into the nucleus, preventing the transcription of AR-responsive target genes.
Our results suggest that the anti-androgen/AR effects of sesquiterpenoids are mediated through a reduction of AR expression, inhibition of AR translocation into the nucleus, reduction of the expression of ARA70 and SRC-1, and interference with the interaction between AR and ARA70 and SRC-1, leading to the inhibition of AR transactivity. Additional studies in animals as well as clinical trials will be necessary to evaluate the potential benefit of utilizing these sesquiterpenoids in the treatment of prostate cancer.
Author contribution
Hui-qing YUAN designed the research; Xiao-ling WANG and Feng KONG performed the research; Tao SHEN and Hong-xiang LOU contributed to the isolation and determination of chemicals; Xiao-ling WANG analyzed the data; and Xiao-ling WANG, Charles YF YOUNG, and Hui-qing YUAN wrote the paper.
References
Feldman BJ, Feldman D . The development of androgen-independent prostate cancer. Nat Rev Cancer 2001; 1: 34–45.
Edwards J, Krishna NS, Grigor KM, Bartlett JM . Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer 2003; 89: 552–6.
Kung HJ, Evans CP . Oncogenic activation of androgen receptor. Urologic Oncology 2009; 27: 48–52.
Dehm SM, Tindall DJ . Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol 2007; 21: 2855–63.
Taplin ME, Balk SP . Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem 2004; 91: 483–90.
Li LF, Chen RS, Liu XM . Xihuangwan induce Bel-7402 cell apoptosis and the change of intracellular-free calcium level in the process. Chin J Clin Hepatol 2003; 19: 362–3.
Xiong Y, Kong XY, Chen RS, Jin QW, Liu XM . The morphological study on the induction of apoptosis in vitro by Chinese herbal medicine Xihuangwan. Chin J Clin Gastroenterol 2001; 13: 82–4.
Ubillas RP, Mendez CD, Jolad SD, Luo J, King SR, Carlson TJ, et al. Antihyperglycemic furanosesquitterpens from Commiphora myrrha. Planta Med 1999; 65: 778–9.
Dolara P, Corte B, Ghelardini C, Pugliese AM, Cerbai E, Menichetti S, et al. Local anaesthetic, antibacterial and antifungal properties of sesquiterpenes from myrrh. Planta Med 2000; 66: 356–8.
Zhu N, Kikuzaki H, Sheng S, Sang S, Rafi MM, Wang M, et al. Furanosesquiterpenoids of Commiphora myrrha. J Nat Prod 2001; 64: 1460–2.
Tipton DA, Lyle B, Babich H . Dabbous MKh . In vitro cytotoxic and anti-inflammatory effects of myrrh oil on human gingival fibroblasts and epithelial cells. Toxicol In Vitro 2003; 17: 301–10.
El Ashry ES, Rashed N, Salama OM, Saleh A . Components, therapeutic value and uses of myrrh. Pharmazie 2003; 58: 163–8.
Shoemarker M, Hamilton B, Dairkee SH, Cohen I, Campbell MJ . In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother Res 2005; 19: 649–51.
Ji K, Kong F, Shen T, Wang XL, Xu AH, Yuan HQ, et al. Separation and identification of myrrh sesquiterpenoids and their anti-proliferation effect on tumor cells. J Shandong Univ (Health Sciences) 2008; 46: 344–8.
Wang XL, Kong F, Ji K, Cai J, Ren K, Gong L, et al. Overexpression of p21WAF/CIP1 is involved in sesquiterpenoids-mediated inhibitory effect on proliferation of prostate cancer cells. Weisheng Dulixue Zazhi 2008; 22: 10–3.
Yuan HQ, Gong AY, Young CYF . Involvement of transcription factor Sp1 in quercetin-mediated inhibitory effect on the androgen receptor in human prostate cancer cells. Carcinogenesis 2005; 26: 793–801.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001; 25: 402–8.
Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD . Targeting the androgen receptor: improving outcomes for castration resistant prostate cancer. Endocr Relat Cancer 2004; 11: 459–76.
Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ . Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res 2002; 62: 1008–13.
Kim J, Coetzee GA . Prostate specific antigen gene regulation by androgen receptor. J Cell Biochem 2004; 93: 233–41.
Mitchell SH, Murtha PE, Zhang S, Zhu W, Young CY . An androgen response element mediates LNCaP cell dependent androgen induction of the hK2 gene. Mol Cell Endocrinol 2000; 168: 89–99.
Heinlein CA, Chang C . Androgen receptor (AR) coregulators: an overview. Endocr Rev 2002; 23: 175–200.
Oñate SA, Tsai SY, Tsai MJ, O'Malley BW . Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 1995; 270: 1354–7.
Rahman MM, Miyamoto H, Takatera H, Yeh S, Altuwaijri S, Chang C . Reducing the agonist activity of antiandrogens by a dominant-negative androgen receptor coregulator ARA70 in prostate cancer cells. J Biol Chem 2003; 278: 19619–26.
Hu YC, Yeh S, Yeh SD, Sampson ER, Huang J, Li P, et al. Functional domain and motif analyses of androgen receptor coregulator ARA70 and its differential expression in prostate cancer. J Biol Chem 2004; 279: 33438–46.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 30772594); Shandong Scientific Reward Funding Program (No 2006BS03006); and the Independent Innovation Foundation of Shandong University, IIFSDU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Xl., Kong, F., Shen, T. et al. Sesquiterpenoids from myrrh inhibit androgen receptor expression and function in human prostate cancer cells. Acta Pharmacol Sin 32, 338–344 (2011). https://doi.org/10.1038/aps.2010.219
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.219
Keywords
This article is cited by
-
The multifaceted therapeutic value of targeting steroid receptor coactivator-1 in tumorigenesis
Cell & Bioscience (2024)
-
Frankincense and myrrh and their bioactive compounds ameliorate the multiple myeloma through regulation of metabolome profiling and JAK/STAT signaling pathway based on U266 cells
BMC Complementary Medicine and Therapies (2020)
-
Efficacy and safety of myrrh in patients with incomplete abortion: a randomized, double-blind, placebo-controlled clinical study
BMC Complementary Medicine and Therapies (2020)
-
Antimicrobial evaluation of novel buccoadhesive films containing Myrrh extract
Polymer Bulletin (2017)
-
Antiproliferative activities of Garcinia bracteata extract and its active ingredient, isobractatin, against human tumor cell lines
Archives of Pharmacal Research (2014)