Abstract
Aim:
To construct an eukaryotic expression vector containing the aldehyde dehydrogenase-2 (ALDH2) gene, and determine whether transfection with the ALDH2 gene can provide protection against hydrogen peroxide-induced oxidative damage, as well as attenuate apoptosis or cell death in human peripheral blood mononuclear cells (PBMCs).
Methods:
The ALDH2 gene was cloned from human hepatocytes by RT-PCR. The eukaryotic expression vector containing the gene was constructed and then transfected into PBMCs via liposomes. RT-PCR, indirect immunofluorescence assay, and Western blot were used to evaluate the expression of the transgene in target cells. MTT assay and flow cytometry were used to detect the effects of ALDH2 on PBMCs damaged by hydrogen peroxide (H2O2). The level of intracellular reactive oxygen species (ROS) was determined by fluorescence spectrophotometry.
Results:
The eukaryotic expression vector pcDNA3.1/myc-His-ALDH2 was successfully constructed and transfected into PBMCs. RT-PCR results showed higher mRNA expression of ALDH2 in the gene-transfected group than in the two control groups (empty vector-transfected group and negative control). Indirect immunofluorescence assay and Western blot indicated distinct higher protein expression of ALDH2 in the gene-transfected group. The cell survival rate against H2O2-induced oxidative damage was higher in the ALDH2 gene-transfected group. Moreover, apoptosis rates in gene-transfected PBMCs incubated with 50 and 75 μmol/L H2O2 decreased by 7% and 6%, respectively. The generation of intracellular ROS was also markedly downregulated.
Conclusion:
ALDH2 gene transfection can protect PBMCs against H2O2-induced damage and attenuate apoptosis, accompanied with a downregulation of intracellular ROS. ALDH2 functions as a protector against oxidative stress.
Similar content being viewed by others
Introduction
The rapid development of medical and molecular biology techniques has resulted in the rise in importance of and focus on tumor immunotherapy research. In 1989, Komori1 reported the first successful infusion of IL-2 activated donor lymphocytes after the recurrence of allogeneic hematopoietic stem cell transplantation (HSCT) for acute lymphoblastic leukemia. Since then, adoptive immunotherapy has continued to show challenging and novel application prospects in the treatment of malignant tumors. In recent years, great progress has been made in clinical tests involving adoptive immunotherapy, and various studies on phase I and II clinical trials have been conducted. Immune cells participate in immune response to protect the body during the therapeutic process, but as first-line cells, they are more vulnerable to oxidative damage2, 3, 4, 5. In different diseases such as renal, cardiovascular, neoplastic, and neurodegenerative illnesses, as well as in aging6, 7, 8, 9, an oxidative stress-induced inflammatory event leading to cellular or tissue injury can be considered a unifying mechanism of injury. Reactive oxygen species (ROS), including hydroxyl radicals, superoxide anions, hydrogen peroxide (H2O2), and singlet oxygen, are generated not only in the process of radiotherapy or chemotherapy before HSCT, but also during therapy that uses drugs that cause DNA damage and induce subsequent apoptosis with the generation of ROS10, 11, 12. Excessive accumulation of ROS can induce oxidative damage to donor or recipient immune cells, weakening the effects of immunotherapy. The protection of immune cells against oxidative damage is, therefore, extremely important to immunotherapy.
At present, many new therapeutic methods are being used in clinical treatments, and a recent strategy involves transferring protective genes in normal immune cells via appropriate vectors to enhance host immunological function against diseases. As a metabolic enzyme of toxic substances, mitochondrial aldehyde dehydrogenase (ALDH2) metabolizes acetaldehyde and plays a major role in the oxidation of acetaldehyde and protection against oxidative injury. It is highly expressed in human liver tissue and lowly expressed in peripheral blood mononuclear cells (PBMCs). It also varies with individual genetic diversity. Some people express inactive ALDH2 for gene mutation. ALDH2 is a nuclear-encoded mitochondrial enzyme that is localized in the mitochondrial matrix, and mitochondria are the major source of ROS. Excessive ROS attacks polyunsaturated fatty acids leading to membrane lipid peroxidation, thereby generating reactive aldehydes, including 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde. ALDH2 provides protection against oxidative damage through the oxidation of exogenous and endogenous aldehydes including 4-HNE, which is the product of lipid peroxidation13, 14, 15. Thus, ALDH2 gene modification may enhance the anti-oxidative stress capacity of immune cells.
This research aims to determine whether the overexpression of ALDH2 can protect human PBMCs against H2O2-induced oxidative damage and attenuate apoptosis. We also investigated the level of ROS in this process. PBMCs are rich in immune cells, their activation and proliferation were studied in this research. This study is a convenient and effective initial foray into investigations on immune cells in gene therapy. Our team previously reported that the resistance to chemotherapeutic agents could be enhanced by transfection with resistant genes in human peripheral blood hematopoietic progenitor cells and PBMCs16, 17. In this study, we explored the protective effect of the ALDH2 gene and provide reference for further related research on gene therapy in practical immunotherapy.
Materials and methods
Construction of eukaryotic expression plasmid
Human ALDH2 cDNA (1.5 kb) was synthesized through reverse transcriptase polymerase chain reaction (RT-PCR) with total RNA extracted from normal human hepatocytes. The ALDH2 cDNA was cloned with DNA polymerase (TaKaRa, Japan) into pBS-T vector (TIANGEN, China) and sent to Jinsite Bio Inc (China) for sequencing. The primers used in PCR were ALDH2 forward 5′-GACACGGATCCATGTTGCGCGCTGCCGCCCGCTTCGG-3′ and ALDH2 reverse 5′-GACACGAATTCTTATGAGTTCTTCTGAGGCACTTTGAC-3′. To construct the eukaryotic expression plasmid expressing the ALDH2 gene pcDNA3.1/myc-His, plasmid (Invitrogen, USA) and pBS-T-ALDH2 were digested with restriction endonucleases EcoR and BamH I, respectively. The refined ALDH2 segments and linearized pcDNA3.1/myc-His were ligated using T4 DNA ligase with a ratio of 2:1 to construct eukaryotic expression plasmid pcDNA3.1/myc-His-ALDH2 (pcDNA3.1(+)-ALDH2). The eukaryotic expression plasmid was verified by PCR and digestion analysis.
Primary culture and activation of PBMCs
PBMCs from the blood of healthy adult volunteers were isolated using a Ficoll lymphocyte separating liquid, and cultivated in an RPMI-1640 culture medium (Gibco, USA) containing 10% fetal bovine serum in 6-well culture plates at 37 °C, 95% O2, and 5% CO2. Phytohemagglutinin (2.5 μg/mL) was added into the culture medium. After 8 h, the cells were moved into fresh medium, into which interleukin-2 (1000 U/mL) was added for activation. The culture medium was replaced with fresh medium 48 h later when the PMBCs were ready for use in the experiments.
Transfection of pcDNA3.1/myc-His-ALDH2 into PBMCs
For eukaryotic expression plasmid transduction, PBMCs were passaged into 6-well plates at a density of 1×106 cells/well. When the cells reached 50% confluence (typically on the third day after subculturing), the medium was replaced with 1 mL fresh medium (without serum) containing 8 μL Lipofectamin 2000 transfection reagent (Invitrogen, USA) and 2 μg plasmid DNA. Cells in group 1 were transfected with pcDNA3.1/myc-His-ALDH2, and group 2 with pcDNA3.1/myc-His empty plasmid. Group 3 was used as the negative control group (without plasmid transfection). The medium was replaced with fresh medium after 4 h. Indirect immunofluorescence assay was used to observe the protein expression of ALDH2 after transfection. Transfection rate was calculated at 12, 24, 48, and 72 h according to the observed fluorescence intensity.
RT-PCR analysis
Total RNA was extracted using TRIzol isolation reagent (TaKaRa, Japan), and RT-PCR analysis was performed using a one-step RT-PCR kit ( Bio Shinegene Inc., China). β-actin was used as internal control. The primers were actin forward 5′-GCTCGTCGTCGACAACGGCTC-3′ and actin reverse 5′-CAAACATGATCTGGGTCATCTTCTC-3′. RT-PCR analysis was performed at 42 °C for 60 min, 92 °C for 5 min, then at 30 cycles of 94 °C for 50 s, 70.5 °C for 60 s, 72 °C for 60 s, and 72 °C for 10 min. An aliquot (5 μL) from the RT-PCR product was subjected to electrophoresis in 1% agarose gel. The anticipated β-actin PCR product was 353 bp long and ALDH2 was 1576 bp.
Western blot analysis
For Western blot analysis, the cells were washed three times with PBS, homogenized in cell lysis buffer, incubated on ice for 20 min, and then centrifuged for 15 min at 10000×g. The aqueous supernatant was collected and quantified using a BCA protein assay kit (Boster, China). Equal amounts (20 μg) of protein extract were loaded and separated by SDS-polyacrylamide gel electrophoresis. After electrophoresis, the proteins on the gel were transferred to polyvinylidene difluoride membranes. The blotted membranes were blocked with 5% skim milk powder in TBS buffer and then probed for 1.5 h with the ALDH2 monoclonal antibody (1:250; H00000217-M01, Abnova, Chinese Taipei) specific for human cellular ALDH2. After three 10-min washing with TBS-Tween 20 buffer (0.05%), the blots were incubated with a secondary antibody (1:3000; Goat Anti-Mouse IgG-HRP Conjugate secondary antibody, Abnova) for 1.5 h. Antibody binding was visualized using an enhanced chemiluminescence reagent (Santa Cruz, USA) according to the manufacturer's instructions.
Cytotoxicity assay
The cytotoxicity of H2O2 to cells in groups 1–3 were examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) test. Briefly, cells from the three groups were seeded into a 96-well cell culture plate at a density of 5×103 cells/well and incubated overnight. The medium was discarded, and new medium containing 1000.000, 100.000, 10.000, 1.000, 0.100, 0.010, 0.001, and 0 μmol/L H2O2 were added into respective wells. Each concentration was tested four times. After 72 h, the medium was discarded and 20 μL of MTT (5 mg/mL) was added to the medium. After another 4 h, the medium was discarded through centrifugation, followed by the addition of DMSO to dissolve the endocellular crystal. OD value was measured at 570 nm on a microplate reader (B10 TEK EL×800UV, General Corporation, USA), with 485 nm as reference. Regression analysis was performed with SPSS followed by the calculation of IC50 values.
Reactive oxygen species analysis
The measurement of intracellular ROS was based on the ROS-mediated conversion of non-fluorescent dichlorofluorescin diacetate (DCFH-DA) into dichlorofluorescin (DCFH). DCFH is a nonfluorescent compound that is oxidized into fluorescent 2, 7-dichlorofluorescin (DCF) in the presence of oxidants, which can be quantified using a fluorescence spectrophotometer. Briefly, 48 h after transfection, 1×106 cells/well on the 6-well plates were harvested after incubation in the presence or absence of H2O2 for 8 h, and then replaced with serum-free medium containing 10 μmol/L DCFH-DA for 20 min at 37 °C. The cells were then rinsed three times with PBS. The fluorescence intensity of the cells was measured using a fluorescence spectrophotometer (Cary Elipse, USA).
Flow cytometric analysis of cell apoptosis
Seventy-two hours after transfection, transfection and expression of the transgene in target cells were evaluated and selected in the medium containing different concentrations of H2O2. For quantitative analysis of cell apoptosis, 1×105 treated cells/tube in 500 μL binding buffer were incubated with Annexin V-FITC (5 μL) and propidium iodide (5 μL) for 5 min at room temperature. The ratio of apoptosis was analyzed by flow cytometry (FCM) (BD FACSCalibur, USA) with CellQuest research software.
Statistical analysis
Each experiment was performed at least three times. All values are expressed as means±SD. Data were collected by the Student's t-test using SPSS13.0. P<0.05 was considered statistically significant.
Results
Construction of eukaryotic expression vector
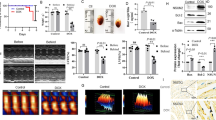
Human ALDH2 was cloned from human hepatocytes by RT-PCR. The PCR optimum temperature was 70.5 °C (Figure 1A). The recombinant eukaryotic expression plasmid pcDNA3.1 (+)-ALDH2 was verified by PCR, sequencing, and endonuclease digestion. The sequencing results of recombinant plasmid showed that the cloned gene was identical to the ALDH2 cDNA published in GeneBank (NM_000690, sequence number Q0468). The fragment digested by endonuclease digestion analysis was about 1560 bp and the amplified fragment by PCR confirmation was 1576 bp, as expected (Figure 1B).
Detection of ALDH2 expression in normal human hepatocytes and recombinated plasmid pcDNA3.1(+)-ALDH2. ALDH2 gene in human hepatocytes was cloned by RT-PCR. (A) Lane M: DNA Marker; Lanes 1–6: RT-PCR-amplified ALDH2 products in different PCR-extending temperatures, Lane 4 was 70.5 °C; Lane 7: negative control; ALDH2 eukaryotic expression vector was verified by PCR and endonuclease digestion; (B) Lane M: DNA marker; Lanes 1, 2: PCR-amplified ALDH2 products (1576 bp) of pcDNA3.1(+)-ALDH2 vectors; Lanes 3, 4: results of endonuclease digestion; Lane 5: PCR-amplified ALDH2 products (1576 bp) of pcDNA3.1(+)-ALDH2 vectors in bacterial liquid; Lane 6: DNA fragment of single enzyme digestion; Lanes 7, 8: circular DNA fragments of pcDNA3.1(+)-ALDH2 and pcDNA3.1(+).
Observation of ALDH2 protein
Creep plates of cultured cells were incubated with an ALDH2 monoclonal antibody and fluorescein isothiocyanate-labeled second antibody. Fluorescence intensity in cells was determined using a fluorescence microscope. ALDH2 proteins expression in group 1 (transgene group) were found 24 h after transfection and became very bright 48 h later (Figure 2). The fluorescence remained bright for at least one week. Cells with weak fluorescence intensity was considered the negative control and those with the brightest density were as the positive control. The transfection efficiency was calculated. The transfection efficiency in PBMCs was (33.5±3.5)% at 24 h, (56.6±2.5)% at 48 h, and 100% at 72 h.
ALDH2 protein expression in PBMCs by indirect immunofluorescence assay. Fluorescence intensity of cells expressing ALDH2 proteins was observed 24 h after transfection. Cells in negative control group at 48 h (A) exhibited weak expression of ALDH2; cells in group 1 (pcDNA3.1(+)-ALDH2 group) at 24 h (B); 48 h (C); and 72 h (D) exhibited considerably stronger fluorescence intensity (P<0.01, n=3). (Original magnification×20 NIKON E5400, F3.9, focal length: 14.5, ISO: 200).
Expression of ALDH2 mRNA
The mRNA expression of ALDH2 in group 1 (transfected with pcDNA3.1(+)-ALDH2) was considerably high, but there was no distinct ALDH2 band between groups 2 (transfected with pcDNA3.1/myc-His) and 3 (negative control group) (Figure 3). These results indicate that the transgene PBMCs had a high mRNA expression of ALDH2.
ALDH2 gene transfection induced over-expression of the mRNA of ALDH2 in PBMCs. Products of RT-PCR were analyzed by agarose gel electrophoresis. Lanes 1, 2, 3: RT-PCR amplified ALDH2 and β-actin products from experimental groups 1–3, respectively. β-actin was used as loading control. ALDH2 mRNA expression was considerably increased in cells transfected with pcDNA3.1(+)-ALDH2, compared with that in untransfected cells (P<0.01 vs control group, n=3).
Expression of ALDH2 protein
Results of Western blot analysis demonstrated that the ALDH2-transfected cells in group 1 expressed a very high level of human ALDH2 protein compared with the empty vector-transfected group and the negative control group because the protein strips were distinct in Lane 1, but indistinct in the other two lanes (Figure 4). These results showed higher protein expression of ALDH2 in the gene-transfected group after being transfected for 72 h. PcDNA3.1/myc-His-ALDH2 was successfully expressed in PBMCs. These results indicate that the transfection of recombinant eukaryotic expression plasmid pcDNA3.1/myc-His-ALDH2 was an effective method for inducing overexpression of ALDH2 in cultured PBMCs.
ALDH2 gene transfection induced over-expression of the protein of ALDH2 in PBMCs. ALDH2 protein expressions were detected by Western blot. β-actin was used as loading control. ALDH2 protein expression considerably increased in cells transfected with pcDNA3.1(+)-ALDH2, compared with that in un-transfected cells (P<0.01 vs control group, n=3).
Effect of ALDH2 on cultured PBMCs injured by H2O2
The relationship between the semilogarithm of H2O2 concentration and cell survival rate was determined by the MTT test (Figure 5). The IC50 of groups 1, 2, and 3 were obtained using the semilogarithmic regressive equation (calculated with SPSS). The values were 8.833, 2.138, and 1.99 μmol/L, respectively. The semilogarithmic regressive equation of groups 1–3 were y=0.6261–0.1333 lg x, y=0.547–0.1424 lg x, and y=0.5413–0.1381 lg x, respectively. The survival rate data indicated that ALDH2-modified PBMCs (group 1) had a higher tolerance for H2O2 than did groups 2 and 3 (P<0.01). There was no significant difference between groups 2 and 3.
Effect of ALDH2 on cultured PBMCs injured by H2O2. Cells were treated with different concentrations of H2O2. Semilogarithmic graphs of concentration-survival rate of PBMCs were drawn based on MTT results (group 1: pcDNA3.1(+)-ALDH2 group; group 2: pcDNA3.1 group; group 3: negative control) (P<0.05, n=4).
Effect of ALDH2 on ROS generation
Treatment with 0, 50, 75, and 100 μmol/L H2O2 on PBMCs of the transgene group for 8 h produced a significant decrease in ROS in group 1 compared with that in groups 2 and 3 (P<0.01 or P<0.05; Figure 6A). There was no significant difference between groups 2 and 3 (P>0.05). ALDH2 transfection markedly decreased intracellular ROS generation, indicating that ALDH2 could prevent the increase of ROS generation induced by H2O2 at 50, 75, and 100 μmol/L (Figure 6B). ALDH2 transfection could decrease intracellular reactive oxygen species (ROS) generation within a certain concentration.
Effect of ALDH2 on the generation of ROS induced by H2O2 in PBMCs. Cells were exposed to 0, 50, 75, and 100 μmol/L H2O2 for 8 h. n=3. Mean±SD. bP<0.05, cP<0.01 vs control. (A) (group 1: pcDNA3.1(+)-ALDH2 group; group 2: pcDNA3.1 group; group 3: negative control). The percentage of ROS level in the transgene group of control group is shown (B). Relative fluorescence intensities were calculated using group 3 cells as control at different concentrations, and a concentration-dependent decrease of ROS in pcDNA3.1(+)-ALDH2 group is shown. bP<0.05 vs control. eP<0.05 vs 0 μmol/L. hP<0.05 vs 50 μmol/L.
Effect of ALDH2 on H2O2-induced apoptosis
Treatment of PBMCs with 0, 50, 75, and 100 μmol/L H2O2 for 12 h resulted in cell apoptosis, which can be evaluated by FCM (Figure 7A). Transgene groups treated with H2O2, however, showed that ALDH2 could attenuate H2O2-induced cell apoptosis within a certain concentration (Figure 7B). ALDH2 had markedly protective effect against H2O2-induced (50, 75 μmol/L) apoptosis on PBMCs in group 1 compared with groups 2 and 3 (P<0.05). ALDH2 transfection could protect cells against H2O2-induced early and advanced apoptosis at certain concentrations.
ALDH2 gene transfection reduced cell apoptosis rate induced by H2O2. Annexin V and PI FACS analysis showed that early- and advanced-stage apoptotic rates decreased in group 1 (A). Treatment with ALDH2 gene transfection markedly decreased apoptosis compared with the two control groups. n=3. Mean±SD. bP<0.05, cP<0.01 vs control. (B) (group 1: pcDNA3.1(+)-ALDH2 group; group 2: pcDNA3.1 group; group 3: negative control).
Discussion
In this study, we demonstrated that the overexpression of ALDH2 protected PBMCs against oxidative damage and considerably attenuated apoptosis. Oxidative damage could be induced by H2O2, and ALDH2 gene transfection enhanced the anti-oxidative effect accompanied with the decreased generation of intracellular ROS in PBMCs.
Adoptive immunotherapy is a new strategy for the treatment of tumors. PBMCs are rich in immune cells, which play a central role in immunotherapy, especially in adoptive immunotherapy. PBMCs are the source of various immune cells, such as natural killer (NK) cells and T lymphocytes. In fact, HSCT is being developed into a type of immunotherapy. Donor CD4+, CD8+, and NK cells have been reported to mediate graft-versus-leukemia effects. Oxidative stress is always induced in different diseases or the pretreatment of radiotherapy and chemotherapy before HSCT. However, immune cells are first subjected to oxidative damage through the generation of redundant ROS18, 19, 20, 21, 22, 23, 24. Considering the improvements in genetic technology, the use of gene modification appears to be an attractive approach for the protection of immune cells against oxidative damage. We propose that ALDH2 could contribute to the protection of PBMCs against oxidative damage and interpret the mechanism of ALDH2 protection, as well as assist in immune therapy. We chose activated PBMCs, which provide abundant unseparated immune cells from healthy volunteers for the study; however, in clinical applications, we used isolated immune cells such as NK cells, which may have presented some limitations.
First, we cloned the ALDH2 gene from normal human hepatocytes and constructed the eukaryotic expression vector pcDNA3.1/myc-His-ALDH2, which was successfully transfected into PBMCs. The ALDH2 strongly affects alcohol metabolism. Inactive ALDH2 is considered contributory to alcohol flushing, preventing people from developing alcoholism25, 26, 27. ALDH2 can also protect cells against damage induced by endogenous and exogenous toxic substances. Several reports have suggested that polymorphism ALDH2 genes have a fundamental relationship with colorectal cancer, primary hepatocellular carcinoma, and stomach cancer28, 29, 30, 31. More important, mitochondrial ALDH2 functions as a protector against oxidative stress14, 32, 33. Our subsequent research verified this viewpoint.
As the inductor, H2O2 is a central oxygen metabolite during the complete reduction of oxygen to H2O. It is a biologically important oxidant due to its ability to generate highly reactive and extremely potent hydroxyl radicals34. The half life of H2O2 is longer than those of other reactive oxygen species; thus, it is typically used to induce oxidative stress in vitro35. PBMCs are sensitive to H2O2-induced oxidative stress. In this study, the treatment of PBMCs with different concentrations of H2O2 induced apoptosis in a concentration-dependent manner. ALDH2 overexpression in PBMCs conferred cellular resistance to H2O2 and considerably attenuated hyperoxia-induced cell apoptosis and death. It was proved that ALDH2 could protect PBMCs against H2O2-induced oxidative damage and apoptosis. The mechanism of these effects is attributed to the metabolism of 4-HNE and the downregulation of intracellular ROS.
In this study, the ROS production in cultured PBMCs was elevated after H2O2 exposure. Overexpression of ALDH2 decreased intracellular ROS production, indicating that ALDH2 might have antioxidant and cytoprotective effects on PBMCs. ROS are the by-products of normal cell metabolism during enzymatic electron-transporting processes, such as mitochondrial respiration, and there is an array of antioxidant systems for maintaining redox balance36. Excessive accumulation of ROS, however, can result in the development of oxidative stress. The majority of ROS are produced in mitochondria, resulting in peroxidation of the mitochondrial membrane. The mitochondrial inner membrane is rich in polyunsaturated fatty acids, such as cardiolipin; thus, reactive aldehydes, including 4-HNE, would be easily derived from peroxidated polyunsaturated fatty acids. Thus, the rapid elimination of 4-HNE is necessary in mitochondria. Evidence supports the toxicity of 4-HNE, which results in injury and cell death through both apoptosis and necrosis37. ALDH2 plays a major role in the oxidation of aliphatic and aromatic aldehydes, which are exogenous or endogenous, including 4-HNE. This study proved that the decrease in intracellular ROS is involved in the mechanism of protective effects, and mitochondrial ALDH2 plays a major role in the clearance of cytotoxic aldehydes derived from peroxides. Moreover, there may be other mechanisms related to these protective effects, such as the activation of Akt, AMP-activated protein kinase, and ERK/MAPK and PI3K-Akt pathways. However, further studies are still needed to shed light on these relationships14, 38.
In summary, this study provides evidence that overexpression of ALDH2 can protect PBMCs against H2O2-induced oxidative damage and attenuate apoptosis. Our results also showed that compared with the control cells, the generation of intracellular ROS in gene-transfected cells markedly decreased after ALDH2 transfection. Mitochondrial ALDH2 functions as a protector against oxidative stress in PBMCs. Gene modification focusing on overexpression of ALDH2 and proteins in the immune cells may help elucidate the mechanism of the protective effect of ALDH2, and suggest potential novel gene therapies to assist in immunotherapy.
Author contribution
Qin FANG, Ji-shi WANG designed the research; Xiu-ying HU performed the research; Jian-qiong XIE, Yuan YANG, Bai-sheng CHAI, and Xin CUI contributed some reagents and assisted to construct eukaryotic expression vector; Fang-qiong LI assisted to construct Western blot; Qin FANG and Xiu-ying HU analyzed data and wrote the paper.
References
Komori T, Sugiyama H, Ogawa H, Oka Y, Miyake S, Soma T, et al. Treatment of a patient in a relapse after bone marrow transplantation for acute lymphoblastic leukemia with the systemic administration of allogeneic lymphokine-activated killer cells and recombinant interleukin-2. Eur J Haematol 1989; 43: 184–5.
Tricarico M, Rinaldi M, Bonmassar E, Fuggetta MP, Barrera G, Fazio VM . Effect of 4-hydroxynonenal, a product of lipid peroxidation, on natural cell mediated cytotoxicity. Anticancer Res 1999; 19: 5149–54.
Schmielau J, Finn O J . Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res 2001; 61: 4756–60.
Mellqvist UH, Hansson M, Brune M, Dahlgren C, Hermodsson S, Hellstrand K . Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: role of reactive oxygen species and regulation by histamine. Blood 2000; 96: 1961–8.
Tseng YM, Chen SY, Chen CH, Jin YR, Tsai SM, Chen IJ, et al. Effects of alcohol-induced human peripheral blood mononuclear cell (PBMC) pretreated whey protein concentrate (WPC) on oxidative damage. J Agric Food Chem 2008; 56: 8141–7.
Kaysen GA, Eiserich JP . The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol 2004; 15: 538–48.
Kraśniak A, Drozdz M, Pasowicz M, Chmiel G, Kowalczyk-Michałek M, Szumilak D, et al. Influence of microinflammation and oxidative stress on atherosclerosis progression and calcifications in cardiovascular system of hemodialyzed patients during two years follow-up. Przegl Lek 2007; 64: 140–7.
Mena S, Ortega A, Estrela JM . Oxidative stress in environmental-induced carcinogenesis. Mutat Res 2009; 674: 36–44.
Uttara B, Singh AV, Zamboni P, Mahajan RT . Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 2009; 7: 65–74.
Mizutani H, Tada-Oikawa S, Hiraku Y, Kojima M, Kawanishi S . Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci 2005; 76: 1439–53.
Rosato RR, Almenara JA, Maggio SC, Coe S, Atadja P, Dent P, et al. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol Cancer Ther 2008; 7: 3285–97.
Fang J, Nakamura H, Iyer AK . Tumor-targeted induction of oxystress for cancer therapy. J Drug Target 2007;15: 475–86.
Li SY, Mark G, Jinhong D . Overexpression of aldehyde dehydrogenase-2 transgene prevents acetaldehyde induced cell injury in human umbilical vein endothelial cells. J Biol Chem 2004; 279: 11244–52.
Xu D, Guthrie JR, Mabry S, Sack TM, Truog WE . Mitochondrial aldehyde dehydrogenase attenuates hyperoxia-induced cell death through activation of ERK/MAPK and PI3K-Akt pathways in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 2006; 291: L966–75.
Ohta S, Ohsawa I . Links dysfunction of mitochondria and oxidative stress in the pathogenesis of Alzheimer's disease: on defects in the cytochrome c oxidase complex and aldehyde detoxification. J Alzheimers Dis 2006; 9: 155–66.
Li DB, Wang JS, Fang Q, Sun HY, Xu W, Li WD . Protective effect of O6-methylguanine-DNA-methyltransferase on mammalian cells. Chin Med J 2007; 120: 714–7.
Wang JS, Fang Q, Sun DJ, Chen J, Zhou XL, Lin GW, et al. Genetic modification of hematopoietic progenitor cells for combined resistance to 4-hydroperoxycyclophosphamide, vincristine, and daunorubicin. Acta Pharmacol Sin 2001; 22: 949–55.
Wu WJ, Pruett SB . Suppression of splenic natural killer cell activity in a mouse model for binge drinking. II. Role of the neuroendocrine system. J Pharmacol Exp Ther 1996; 278: 1331–9.
Collier SD, Wu WJ, Pruett SB . Ethanol suppresses NK cell activation by polyinosinic-polycytidylic acid (poly I:C) in female B6C3F1 mice: role of endogenous corticosterone. Alcohol Clin Exp Res 2000; 24: 291–9.
Li W, Lidebjer C, Yuan XM, Szymanowski A, Backteman K, Ernerudh J, et al. NK cell apoptosis in coronary artery disease: relation to oxidative stress. Atherosclerosis 2008; 199: 65–72.
Rinaldi M, Tricarico M, Bonmassar E, Parrella P, Barrera G, Fazio VM . Effect of 4-hydroxynonenal, a product of lipid peroxidation, on NK susceptibility of human K562 target cells. Anticancer Res 1998; 18: 3591–5.
Thorén FB, Romero AI, Hermodsson S, Hellstrand K . The CD16-/CD56bright subset of NK cells is resistant to oxidant-induced cell death. J Immunol 2007; 179: 781–5.
Carter RH . B cells in health and disease. Mayo Clin Proc 2006; 81: 377–84.
Baraldo S, Lokar Oliani K, Turato G, Zuin R, Saetta M . The role of lymphocytes in the pathogenesis of asthma and COPD. Curr Med Chem 2007; 14: 2250–6.
Nishiyori A, Shibata A, Ogimoto I, Uchimura N, Egami H, Nakamura J, et al. Alcohol drinking frequency is more directly associated with alcohol use disorder than alcohol metabolizing enzymes among male Japanese. Psychiatry Clin Neurosci 2005; 59: 38–44.
Vasiliou V, Pappa A . Polymorphisms of human aldehyde dehydrogenases. Consequences for drug metabolism and disease. Pharmacology 2000; 61: 192–8.
Guo R, Zhong L, Ren J . Overexpression of aldehyde dehydrogenase-2 attenuates chronic alcohol exposure-induced apoptosis, change in Akt and Pim signalling in liver. Clin Exp Pharmacol Physiol 2009; 36: 463–8.
Gao CM, Takezaki T, Wu JZ, Zhang XM, Cao HX, Ding JH, et al. Polymorphisms of alcohol dehydrogenase 2 and aldehyde dehydrogenase 2 and colorectal cancer risk in Chinese males. World J Gastroenterol 2008; 14: 5078–83.
Ding J, Li S, Wu J, Gao C, Zhou J, Cao H, et al. Alcohol dehydrogenase-2 and aldehyde dehydrogenase-2 genotypes, alcohol drinking and the risk of primary hepatocellular carcinoma in a Chinese population. Asian Pac J Cancer Prev 2008; 9: 31–5.
Zhang FF, Hou L, Terry MB, Lissowska J, Morabia A, Chen J, et al. Genetic polymorphisms in alcohol metabolism, alcohol intake and the risk of stomach cancer in Warsaw, Poland. Int J Cancer 2007; 121: 2060–4.
Ren J, Babcock SA, Li Q, Huff AF, Li SY, Doser TA . Aldehyde dehydrogenase-2 transgene ameliorates chronic alcohol ingestion-induced apoptosis in cerebral cortex. Toxicol Lett 2009; 187: 149–56.
Ohta S, Ohsawa I, Kamino K, Ando F, Shimokata H . Mitochondrial ALDH2 deficiency as an oxidative stress. Ann N Y Acad Sci 2004; 1011: 36–44.
Ohsawa I, Kamino K, Nagasaka K, Ando F, Niino N, Shimokata H, et al. Genetic deficiency of a mitochondrial aldehyde dehydrogenase increases serum lipid peroxides in community-dwelling females. J Hum Genet 2003; 48: 404–9.
Clarkson PM, Thompson HS . Antioxidants: what role do they play in physical activity and health. Am J Clin Nutr 2000; 72: 637S–46S.
Fatokun AA, Stone TW, Smith RA . Hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells: the effects of glutamate and protection by purines. Bone 2006; 39: 542–51.
Ohsawa I, Nishimaki K, Yasuda C, Kamino K, Ohta S . Deficiency in a mitochondrial aldehyde dehydrogenase increases vulnerability to oxidative stress in PC12 cells . J Neurochem 2003; 84: 1110–7.
Kakkar P, Singh BK . Mitochondria: a hub of redox activities and cellular distress control. Mol Cell Biochem 2007; 305: 235–53.
Ma H, Li J, Gao F, Ren J . Aldehyde dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol: role of protein phosphatase and forkhead transcription factor. J Am Coll Cardiol 2009; 54: 2187–96.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No 30460127, 30760276, and 81070444).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, Xy., Fang, Q., Wang, Js. et al. Over-expression of aldehyde dehydrogenase-2 protects against H2O2-induced oxidative damage and apoptosis in peripheral blood mononuclear cells. Acta Pharmacol Sin 32, 245–252 (2011). https://doi.org/10.1038/aps.2010.203
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.203
Keywords
This article is cited by
-
Tamoxifen decreases ovarian toxicity without compromising cancer treatment in a rat model of mammary cancer
BMC Genomics (2023)
-
Peripheral blood transcriptomic clusters uncovered immune phenotypes of asthma
Respiratory Research (2022)
-
Alda-1, an Aldehyde Dehydrogenase 2 Agonist, Improves Cutaneous Wound Healing by Activating Epidermal Keratinocytes via Akt/GSK-3β/β-Catenin Pathway
Aesthetic Plastic Surgery (2022)
-
Effects of the common polymorphism in the human aldehyde dehydrogenase 2 (ALDH2) gene on the lung
Respiratory Research (2017)
-
HPV seropositivity joints with susceptibility loci identified in GWASs at apoptosis associated genes to increase the risk of Esophageal Squamous Cell Carcinoma (ESCC)
BMC Cancer (2014)