Abstract
Aim:
To investigate the effects of the cardiotonic steroid, ouabain, on cardiac differentiation of murine embyronic stem cells (mESCs).
Methods:
Cardiac differentiation of murine ESCs was enhanced by standard hanging drop method in the presence of ouabain (20 μmol/L) for 7 d. The dissociated ES derived cardiomyocytes were examined by flow cytometry, RT-PCR and confocal calcium imaging.
Results:
Compared with control, mESCs treated with ouabain (20 μmol/L) yielded a significantly higher percentage of cardiomyocytes, and significantly increased expression of a panel of cardiac markers including Nkx 2.5, α-MHC, and β-MHC. The α1 and 2- isoforms Na+/K+-ATPase, on which ouabain acted, were also increased in mESCs during differentiation. Among the three MAPKs involved in the cardiac hypertrophy pathway, ouabain enhanced ERK1/2 activation. Blockage of the Erk1/2 pathway by U0126 (10 μmol/L) inhibited cardiac differentiation while ouabain (20 μmol/L) rescued the effect. Interestingly, the expression of calcium handling proteins, including ryanodine receptor (RyR2) and sacroplasmic recticulum Ca2+ ATPase (SERCA2a) was also upregulated in ouabain-treated mESCs. ESC-derived cardiomyocyes (CM) treated with ouabain appeared to have more mature calcium handling. As demonstrated by confocal Ca2+ imaging, cardiomyocytes isolated from ouabain-treated mESCs exhibited higher maximum upstroke velocity (P<0.01) and maximum decay velocity (P<0.05), as well as a higher amplitude of caffeine induced Ca2+ transient (P<0.05), suggesting more mature sarcoplasmic reticulum (SR).
Conclusion:
Ouabain induces cardiac differentiation and maturation of mESC-derived cardiomyocytes via activation of Erk1/2 and more mature SR for calcium handling.
Similar content being viewed by others
Introduction
Embryonic stem cells (ESCs) have the ability to self-renew and differentiate into virtually all cell types of the three embryonic germ layers including cardiomyocytes. They thus represent an unlimited ex vivo cell source for cardiac regenerative therapy1, 2, 3, 4 as well as an ideal in vitro model to investigate complex developmental processes. Spontaneous differentiation of ESCs towards cardiac lineage is generally poor1, 5, 6. To date, protocols have exploited transcription factors involved in embryonic heart development to direct ESC differentiation into cardiomyocytes5, 6, 7, 8. In contrast, transcription pathways crucially involved in post-natal hypertrophic growth of cardiomyocytes have not been investigated to improve the efficacy of cardiac differentiation of ESCs.
The cardiotonic glycoside ouabain is a specific inhibitor of the ubiquitous Na+/K+-ATPase that is responsible for the active transport of Na+ and K+ across the plasma membrane of most animal cells. In adult cardiomyocytes, Na+/K+-ATPase inhibition results in a modest increase in intracellular Na+, sufficient to affect the sarcolemmal Na+/Ca2+ exchange and cardiac contractility9, 10, 11. Alterations in concentrations of endogenous cardiotonic glycosides have been reported in various human conditions such as essential hypertension12, asymptomatic left ventricular dysfunction13 and dilated cardiomyopathy14. In experimental models, cardiotonic glycoside has cardioprotective effects against ischemia not associated with Na+/K+-ATPase inhibition15, 16. They also cause transcriptional regulation of several cardiac-growth related genes resulting in hypertrophy of adult cardiomyocytes17. Extensive subsequent studies of various cell types have revealed that binding of cardiotonic glycosides to Na+/K+-ATPase in fact activates multiple pathways including cytoplasmic tyrosine kinase Src/epidermal growth factor receptor (EGFR)18, phosphatidylinositol 3-kinase (PI3K)-Akt19, phospholipase C kinase, and increased mitochondrial production of reactive oxygen species20. The downstream signaling pathway nonetheless appears to be cell-type specific. A previous report demonstrated the functional expression of Na+/K+-ATPase in undifferentiated ESCs as well as ESC-derived cardiomyocytes5 although the effect of ouabain on cardiac differentiation and maturation of ESCs remains unclear. The aims of the present study were thus to determine whether ouabain, the prototypic Na+/K+-ATPase inhibitor and potent hypertrophic stimulus of adult cardiomyocytes, affects cardiac differentiation and maturity of ESCs. In order to validate the cardiac differentiation of ESCs, we quantified the number of troponin-positive cells using flow cytometry and the expression of a panel of cardiac specific markers in differentiated ESCs. We also studied the effects of ouabain on the extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal protein kinase (JNK), and p38 mitogen activated protein kinase (MAPK) during cardiac differentiation of ESCs. Treatment with a specific MAPK inhibitor would be useful to investigate the specific role of MAPK in cardiac differention. In addition, the maturity of calcium handling properties of differentiated ESCs was assessed using confocal calcium imaging.
Materials and methods
Murine embryonic stem cell culture and in vitro cardiac differentiation
Murine (m) ES cell-line D3 (CRL-1934, American Type Culture Collection, Manassas, VA) was used and cultured as previously described21. Briefly, undifferentiated mESCs were cultured on an irradiation-inactivated monolayer of mouse embryonic fibroblast feeders in Dulbecco's modified Eagle's minimal essential medium (DMEM, Gibco BRL, Karlsruhe, Germany), supplemented with 15% fetal bovine serum (FBS, Gibco BRL, Karlsruhe, Germany), 0.1 mmol/L mercaptoethanol (Sigma-Aldrich, St Louis, MO), non-essential amino acids (stock solution diluted 1:100; Hyclone, Logan, UT) and 1000 U/mL of recombinant mouse leukemia inhibitory factor (LIF) (Chemicon, Hofheim, Germany). To induce cardiac differentiation, embryoid bodies (EBs) were generated from hanging drops of approximately 800 mESCs in 20 μl of culture medium in the absence of leukemia inhibitory factor and feeder cells for two days and then grown in suspension or five more days 21.
Effect of cardiotonic glycoside, ouabain, on cardiac differentiation
Embryoid bodies were plated on gelatin-coated plate following five day suspension and cultured with 20 μmol/L ouabain dissolved in phosphate-buffered saline (PBS) at a stock concentration of 10 mmol/L (Sigma-Aldrich, St Louis, MO) for a further seven days. No drug or vehicle was added in the control group. When counting the number of beating EBs, number of day refers to time from plating of EBs onto gelation-coated plates. For Erk1/2 inhibitory experiments, EBs were treated with U0126 in combination with ouabain for 7 days before FACS counting of cardiomycytes. Some EBs pretreated with 20 μmol/L ouabain for 7 days were further incubated with 10 μmol/L U0126 (Cell-Signaling Technology, Danvers, MA) (dissolved in DMSO) in serum-free condition for 2 h prior to harvest for Western blotting. All experiments were performed using EBs generated from different passages of <10. Medium with corresponding drug was refreshed every 2 to 3 days.
Cell viability
Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-y)-2,5–diphenyl-tetrazolium bromide (MTT) staining method described by Mosmann T and Hansen in a 96-well microtiter plate22. This method is based on the ability of viable, but not dead cells, to convert MTT to a blue colored formazan. Stock MTT solution (5 mg/mL) was prepared in PBS. Ten EBs were plated onto each 96-well coated plate with 0.1% gelatin following 5-day suspension and differentiation medium served as control. At the end of incubation, 20 μL of MTT solution was added to each well containing 180 μL medium with various concentrations of ouabain. Following 4 h incubation at 37 °C, dark crystals formed and the reaction was stopped by adding 100 μL of DMSO. The optical density (OD) of each well was read on a Bio-Rad 550 Microplate Reader (Bio-Rad Laboratories, Hercules, CA) at 570 nm. The viability values obtained in the presence of various dosages of ouabain were subsequently normalized against control values.
Isolation of beating mESC-derived cardiomyocytes
The beating outgrowths were microsurgically dissected with a glass knife from D3 mESC-derived 7-day differentiated EBs, and incubated in collagenase B (1 mg/mL) with DNase I (60 U/mL, Roche Applied Sciences Penzberg, Germany) at 37 °C for 30 min with occasional dispersion by pipetting up and down. Isolated cells were recovered in Kraftbrühe (KB) solution containing (mmol/L) 85 KCl, 30 K2HPO4, 5 MgSO4, 1 EGTA, 2 Na2-ATP, 5 pyruvic acid, 5 creatine, 20 taurine, and 20 D-glucose at room temperature for 1 h. The cells were subsequently plated on 0.1% gelatin coated glass cover slips in 24-well culture plates with corresponding differentiation medium. Calcium imaging of isolated cells or cell clusters was performed within 2 days; some of the cells were fixed in 4% paraformaldehyde for immunocytological staining at 4 °C.
Immunocytological staining
The 7-day differentiated EBs were microdissected and fixed with 2% paraformaldehyde for 20 min at 4 °C, followed by washing with wash buffer [DPBS with 0.1% Triton X-100 (Sigma-Aldrich, St Louis, MO)] once for 5 min. Cells were incubated overnight at 4 °C with anti-troponin-T (1:100, Lab Vision, Fremont, CA) primary antibodies, then rinsed three times for five minutes with wash buffer. Further incubation for one hour at room temperature was performed with secondary antibodies, goat anti-mouse FITC antibodies (1:100, Molecular Probes), diluted in wash buffer. The cells were rinsed three times, counterstained and mounted with SlowFade® Gold antifade reagent with DAPI (Invitrogen, Life Technologies, Carlsbad, CA). Fluorescent immunostaining for troponin-T was examined and photographed under a fluorescent microscope (green). The images of troponin-T positive cells were captured by Laser Scanning Systems LSM 510 (Carl Zeiss, Inc, Oberkochen, Germany).
Assessment of cardiac differentiation using reverse transcription-polymerase chain reaction
Total RNA from 7-day old EBs was extracted with Trizol® reagent (Invitrogen, life technologies, Carlsbad, CA). Reverse transcription was then performed using 1 μg of total RNA in a final volume of 20 μL, using the QuantiTect® reverse transcription kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Cardiac-specific genes, alpha isoforms of sodium-potassium ATPase and calcium handling proteins were compared in EBs in the presence or absence of ouabain with quantitative real-time polymerase chain reaction (qPCR). Primer sequence and annealing temperature are depicted in Table 1. GAPDH served as an internal control. Quantitative PCR analysis was performed with a real-time PCR Detector (Opticon 2 DNA Engine, MJ Research, MN, USA) using the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). For amplification, after initial holds for 5 min at 95 oC, 50 cycles of 95 oC for 15 s followed by 57 oC for 30 s and 72 oC for 30 s, melt curve analysis was performed. The relative quantification of PCR products was performed according to the 2-ΔΔCt method, using mouse GAPDH as an internal control. Where ΔΔCt=[(Cttarget gene–CtGAPDH)control group–(Cttarget gene–CtGAPDH)ouabain group].
Confocal calcium imaging
mESC-derived cardiomyocytes were loaded with 1:1 (v/v) amount of 20% Pluronic®-F127 (Invitrogen, Life Technologies) and 5 μmol/L Fluo-3 AM (Sigma-Aldrich, St Louis, MO) dissolved in DMSO with stock concentration of 5 mmol/L for 45 min at 37 °C in Tyrode solution consisting of (mmol/L): 140 NaCl, 5 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose and 10 HEPES at pH 7.4. The calcium transient of single ESC-derived cardiomyocytes was recorded with a confocal imaging system (Olympus Fluoview System version 4.2 FV300 TIEMPO) mounted on an upright Olympus microscope (IX71) with temporal resolution of the line scan at 274 frames per second (2000 scan per 7.3 s). They were then quantified as the background subtracted fluorescence intensity changes normalized to the background subtracted baseline fluorescence using Image J. The amplitude, maximal upstroke and decay velocity of calcium transient were analyzed by Clampfit version 9.2.0.09. (Axon Instruments, Inc, Foster City, CA).
Quantification of cardiac differentiation by flow cytometry
Beating clusters first appeared on day 2 after plating. The percentage of mESC-derived cardiomyocytes was quantified by FACS analysis on day 7 of mESC differentiation. Briefly, 10-cm dishes of EBs were dissociated to a single-cell suspension by collagenase B (1 mg/mL) with DNase (60 U/mL) (Roche Applied Sciences, Penzberg, Germany) treatment, and washed by DPBS twice. Cells were permeabilzed for 15 min using a Cytofix/Cytoperm permeabilization kit (BD Biosciences, San Diego, CA), treated with 1% fetal bovine serum to block non-specific antigens and incubated overnight at 4 °C. Cells were then stained with monoclonal anti-Troponin T [dilution 1:100; Cat no: MS-295-P0; Cardiac Isoform Ab-1 (Clone 13–11)], NeoMarker, Fremont, CA) for 1 h. After twice rinsing in washing buffer, anti-mouse IgG H+L-PE was used for secondary antibody staining (dilution 1:100; Beckman Coulter, Fullerton, CA, USA) for one more hour. Analysis was performed with a Beckman Coulter FC500 flow cytometer in which 10 000 events were counted. The background signal was determined using IgG1 isotypic control as the primary antibody.
Measurement of ERK1/2, JNK, and p38 Western blot analysis
Cells were washed with PBS, and collected in RIPA buffer (Cell Signaling Technology, Danvers, MA) containing 0.2% Triton X-100, 5 mmol/L EDTA, 1 mmol/L PMSF, 10 μg/mL leupeptin, 10 μg/mL aprotinin, with additional 100 mmol/L NaF and 2 mmol/L Na3VO4 and lysed for 30 min on ice. Protein assay was performed using a Bio-Rad protein assay kit (Hercules, CA): 20 μg of protein was loaded per well on a 12.5% sodium dodecyl sulphate (SDS)-polyacrylamide gel, Proteins were subsequently transferred onto 0.45 m pore size nitrocellulose membranes and blocked with 5% non-fat dry milk in TBS (pH 7.4) with 0.5% Tween-20 at 4 °C. The blots were challenged with primary antibody (1:1000) overnight at 4 °C, followed by washing three times with TBST (0.1% Tween-20), then challenged with HRP-conjugated goat anti-rabbit (dilution 1:2000; Cell Signaling Technologies) respectively, followed by detection with enhanced chemiluminescent substrate (Millipore, Billerica, MA). As primary antibodies, the rabbit polyclonal anti-p38 MAPK, anti-ERK, anti-JNK, directed against the phosphorylated form of the proteins (dilution 1:1000; Cell Signaling Technology, Danvers, MA).
Statistical analysis
Continuous variables are expressed as mean±standard deviation. Statistical comparisons were performed using Student's t test. Calculations were performed with SPSS (version 14.0). A P value <0.05 was considered statistically significant.
Results
Ouabain enhanced cardiac differentiation of mESCs
The effect of ouabain on the viability of differentiating mESCs was determined by MTT assay at different concentrations of ouabain for 72 h. Ouabain was well tolerated even in relatively high concentrations in differentiating mESCs (Figure 1A). To assess whether ouabain treatment enhances cardiac differentiation of mESCs and to determine the optimal range ouabain dosage in enhancing cardiac differentiation, flow cytometry to determine the percentage of cardiomyocytes as identified by troponin-T positive cells was performed. As depicted in Figure 1B and 1C, the optimal dosage of ouabain to enhance cardiac differentiation of mESC was about 10 μmol/L (Figure 1A and 1B). Taken together with the viability test, ouabain at concentration of 20 μmol/L was selected for subsequent experiments. Cardiac differentiation of undifferentiated mESCs was assessed by the percentage of spontaneous beating EBs under the microscope and troponin-T positive cells using flow cytometry at d 7. In this study, spontaneous beating outgrowths from EBs were first observed on d 2 in both ouabain-treated EBs and controls (12.5%), and progressively increased until reaching a plateau at d 9. The administration of ouabain resulted in a higher percentage of spontaneous beating outgrowths of EBs compared with controls from d 6 to d 9 (Figure 1B). Standard, counting of beating outgrowths from EBs is nevertheless a very crude measurement of the efficiency of cardiac differentiation4. The percentage of cardiomyocytes was consistently significantly higher in the ouabain group (9.50%±1.82% vs 2.90%±0.20%; n=3, P<0.05).
Enhanced in vitro cardiac differentiation with ouabain. (A) The effect of ouabain on the viability of differentiating D3 mESCs. The EBs were plated and exposed to various dosages of ouabain for 72 h and the viability of cells determined by MTT assay. Data are expressed as mean±SEM (n=6). (B) Dot plots of the percentage of mESC-derived cardiomyocytes (troponin-T positive cells) as determined by flow cytometry at various concentrations of Ouabain. (C) Bar chart of the Troponin-T positive cell counts. (D) Effect of ouabain on percentage of beating clusters of the differentiated ESCs. Number of day is defined as time beating cluster shown after plating of suspended EBs. Data are expressed as mean±SEM (n=3), whereas the significant difference was tested between ouabain-treated and control for each time point by unpaired t-test, bP<0.05.
Figure 2 shows the immunocytochemical pattern of cardiac-specific cytoskeletal proteins including troponin-T in mESC-derived cardiomyocytes. Cardiomyocytes derived from controls showed a homogeneous distribution of troponin-T protein resembling early stage cardiomyocytes (Figure 2)23; cardiomyocytes from the ouabain group demonstrated striations of the myofilament specific protein indicating sarcomere development, a marker for late-stage cardiomyocytes.
Ouabain-induced expression of cardiac specific genes
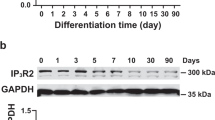
The effect of ouabain on the expression of cardiac marker genes in EBs was examined by quantitative RT-PCR. The expression of cardiac transcription factors, including Nkx2.5, significantly increased (almost double) in the ouabain group on d 7 (n=3, P<0.05), but not GATA-4 and GATA-6, the transcription factors common for mesoderm and endoderm (Figure 3A). In addition, gene expression of sarcomeric muscle proteins (α-MHC and β−MHC) was increased in the ouabain group compared with the control (n=3; P<0.05 and P<0.01 respectively). Of note, the expression of α-MHC, the adult isoform of myosin heavy chain, was markedly up-regulated with ouabain treatment by about 3.5-fold (Figure 3A). The authenticity of increase α-MHC gene expression was confirmed with Western blot experiment (Figure 3B). As a potential target of ouabain, the expression kinetics of α subunit isoforms of Na+/K+ ATPase in mESCs during differentiation was also investigated using RT-PCR. The mRNA of all three isoforms: α1, α2, and α3 subunits was detected in both undifferentiated mESCs and mESC-derived cardiomyocytes at 7-day differentiation; nonetheless α1 and 2 isoforms were the most responsive to ouabain treatment (Figure 3C). In addition, mRNA expression of a panel of calcium handling proteins, including RyR2 and SERCA2a, (Figure 3D) was up-regulated in the ouabain group.
(A) Cardiac maker gene expression of mESC with ouabain-induced cardiac differentiation as revealed by quantitative RT-PCR. Nkx2.5, NK2 transcription factor related locus 5; GATA 4 and 6, GATA-binding protein 4 and 6; MLC2V, myosin light chain 2 ventricular transcripts; α- and β-MHC, α- and β-myosin heavy chain. GAPDH was used as internal control. (B) Western blot of cardiac cytoskeletal protein, alpha-MHC in differentiated mESCs. (C) Gene expression of three isoforms of sodium-potassium ATPase (Na+/K+ ATPase), α1, α2, and α3. (D) Gene expression of calcium handling proteins on sacroplasmic recticulum (SR). Three different experiments were repeated with similar results. RyR2, ryanodine receptor 2; SERCA2a, sarcoplasmic reticulum Ca2+ ATPase; NCX-1: Na/Ca exchanger. GAPDH was used as internal control. Data shown as mean±SEM from 3 independent experiments, significance difference was tested by unpaired t-test with bP<0.05 and cP<0.01.
Ouabain-enhanced calcium handling of cardiomyoyctes derived from mESCs
To investigate whether up-regulation of calcium handling proteins in cardiomyocytes from the ouabain group was associated with more mature calcium handling properties, spontaneous calcium oscillations in single cardiomyoyctes isolated from ouabain-treated EBs were characterized on d 7 using confocal laser microscopy and compared with that of the control. Consistent with the up-regulated calcium handling proteins, the ouabain group exhibited more mature calcium handling properties (Figure 4A). Specifically, cardiomyocytes from the ouabain group generated larger calcium transients [14.52±4.45 (n=7) vs 6.86±0.87 (n=7)], as well as a significantly higher maximal and decay velocity (n=7; P<0.01 and P<0.05 respectively), suggestive of a more mature sarcoplasmic reticular (SR) function (Figure 4B–4D). Administration of caffeine (10 mmol/L) also elicited a significant larger surge in cytosolic Ca2+ in the ouabain group (1163±213.9 vs 612.4±99.7; P<0.05), indicating a substantial increase in SR calcium content conferred by ouabain treatment (Figure 5).
(A) Representative tracings of rythmic spontaneous Ca2+ transients showing in ouabain treated ESC-CM (right) and control (left). (B) Amplitude, (C) Maximal upstroke velocity (Vmax upstroke), (D) Maximal decay velocity (Vmax decay) of Ca2+ transients in the mESC-derived cardiomyocytes; Data shown as mean±SEM (n=7), seven cells of interest were selected from in three independent experiments. Unpaired t-test was performed between ouabain treated and control group; bP<0.05, cP<0.01.
(A) Representative tracings of caffeine-induced SR Ca2+ release in control (left) and ouabain-treated (right) mESC-CM, demonstrating caffeine-sensitive Ca2+ stores and fractional release of total SR Ca2+ load during spontaneous activation. (B) Amplitude, (C) Maximal upstroke velocity (Vmax upstroke), (D) Maximal decay velocity (Vmax decay) of Ca2+ transients in the mESC-derived cardiomyocytes; Data shown as mean±SEM (n=5), five cells were analyzed in three independent experiments. Unpaired t-test was performed between ouabain treated and control group; bP<0.05.
Ouabain increases cardiac differentiation of mESCs by Erk1/2 activation
Various MAPK-signaling cascades including ERK, c-JNK and p38 MAPK play important roles in cardiac hypertrophy17, 24 and cardiac remodeling following myocardial infarction. In order to determine whether the enhanced cardiac differentiation on administration of ouabain is related to MAPK-signaling cascades involved in the hypertrophy pathway, phosphorylation of ERK, c-JNK, and p38 MAPK in ouabain-treated EBs was determined and compared with controls. On d 7, ouabain treatment resulted in a significant surge in phosphorylation of ERK1/2 (Figure 6A); no significant difference between the ouabain group and control was found in phosphorylation of either JNK1/2 or p38 MAPK (Figure 6B). The MEK1/2 inhibitor U0126 suppressed ouabain-activated tyrosine phosphorylation of ERK1/2 (Figure 7A), indicating that ERK1/2 was likely activated by ouabain stimulation of MEK1/2 activity. To further study the effect of Erk1/2 activation on cardiac differentiation of mESCs, FACS experiments were performed by blocking the Erk1/2 pathway. Addition of the MEK1 inhibitor, U0126, which subsequently blocks MEK1/2, upstream of ERK1/2, suppressed the percentage of troponin-T positive cells to 0.53%±0.28% (n=3, P<0.01 compared with control) (Figure 7) while ouabain rescued the effect to control levels (n=3, P<0.05 compared with U0126).
Expression of major MAPK, ERK1/2, p38 and JNK, involved in hypertrophy pathway of mESCs. (A) Ouabain increased Erk1/2 and MEK1/2 phosphorylation and rescued the suppression by U0126; (B) Unchanged phosphorylation of p38 and JNK upon ouabain treatment; at least three independent experiments were repeated with a similar result for each of the MAPK examined.
Mechanistic study on the relative number of ESC-derived cardiomyocytes calculated as the percentage of troponin-T positive cells on d 7 as determined by flow cytometry. (A) Dot plots and (B) bar chart of the troponin-T positive cell counts. Data of independent experiments were expressed as mean±SEM (n=3, bP<0.05, cP<0.01). Significant difference was analyzed by unpaired t-test.
Discussion
We evaluated the effects of ouabain on cardiac differentiation of mESCs. Our results demonstrate that ouabain promotes cardiogenesis and myofibrillogenesis of ESCs, and matures the calcium handling properties of cardiomyocytes derived therefrom. The main findings are as follows: 1) Ouabain-treated EBs showed an increased differentiation into cardiomyocytes (in terms of the percentages of beating outgrowths and troponin-positive cardiomyocytes), probably via Erk1/2 activation; 2) Ouabain increased gene expression of cardiogenesis (Nkx2.5) and myofibrillogenesis (α-MHC and β-MHC); 3) Ouabain increased the mRNA level of calcium handling protein (RyR2 and SERCA2a) with corresponding maturation of calcium handling properties as determined by confocal microscopy; 4) Ouabain significantly enhanced expression of the α1 isoform of Na+/K+-ATPase, the predominant form in cardiac tissue.
ESC-derived cardiomyocytes hold great promise for cardiac regeneration. Nonetheless current protocols for cardiac differentiation of ESCs are in general inefficient, making it very difficult to obtain adequate numbers of cardiomyocytes for clinical therapy. Despite the well-known hypertrophic effects of ouabain on cardiomyocytes17, 24 and the documented functional expression of Na+/K+-ATPase in undifferentiated mESCs5, the potential procardiogenic effects of ouabain have not been explored. Binding of ouabain to Na+/K+-ATPase, in addition to the positive inotropic effect, also activates multiple MAPK pathways in a cell-type specific manner. Previous studies have suggested that MAPK activation may play a crucial role in mesoderm induction, which leads subsequently to cardiogenesis during embryonic development25, 26. Coordinated activation of the three major MAPKs involved in cardiac hypertrophy namely ERK1/2, JNK, and p38 MAPK, are essential to induce cardiac differentiation of P19 embryonic carcinomal cell line27, 28. In the present study, application of ouabain to undifferentiated mESCs resulted in a modest increase in the number of troponin-positive cells differentiated from mESCs. This was associated with increased expression of early cardiac specific transcription factors (Nkx2.5) and cardiac specific markers (α-MHC and β-MHC). Consistent with a previous study29, ERK1/2, JNK and p38 MAPK were endogenously activated in mESCs during differentiation. Only ERK1/2 though was significantly activated upon ouabain treatment. Since specific blocker for ERK1/2 is not available, U0126, an upstream MEK1/2 blocker, were used to study the pathway, of which ouabain induced cardiogenesis. Due to the non-specificity of MEK1/2 blocker, it is possible that other mechanisms independent of ERK1/2 activation may be involved in ouabain induced cardiac differentiation in mECSs. Nonetheless, the specific role of ouabain in Erk1/2 activation was well defined by the inhibitor experiment. Interestingly, various cytokines or growth factors, including cardiotrophin-130, VEGF31, and heregulin-β18, which promote cardiac differentiation of mESCs, also mediate via the activation of ERK1/2 pathway. It has recently been shown that icariin, the active ingredient of the plant herb Epimedium, significant enhances cardiac differentiation of mESCs via activation of p38 MAPK29. Taken together, this evidence suggests that adult cardiac hypertrophic signals, particularly the MAPK pathway, may play a role in the cardiac differentiation in ESCs, and may be exploited to improve the efficiency of cardiac differentiation.
Another important hurdle for ESC-based cardiac therapies is the relative immature calcium handling properties of ESC-derived cardiomyocytes2, 32, 33, 34. In adult cardiomyocytes, calcium enters the cell through L-type calcium channels during phase 2 of action potentials. This relative small calcium influx in turn triggers a large calcium release from the internal calcium store, SR through ryanodine receptors35. This process is known as calcium-induced calcium release (CICR), the primary mechanism that links electrical excitation and mechanical contraction in cardiomyocytes. During diastole, calcium is actively removed from cytosol, mainly through sarco/endoplasmic reticulum Ca2+-ATPase pump (SERCA), back into the SR and via Na+-Ca2+ exchanger (NCX) out of cell36. mESC-derived cardiomyocytes are known to exhibit immature calcium dynamics: small cytosolic calcium transient amplitudes, slow rise and decay kinetics, and reduced calcium content of SR. This adversely affects excitation-contraction coupling37 and is partly related to the relatively underdeveloped SR and partly to the developmental expression profiles of calcium handling proteins in mESC-derived cardiomyocytes. In the present study, ouabain treatment favorably altered the calcium handling properties of mESC-derived cardiomyocytes including larger calcium transients, a faster rate of rise and decay of calcium transients, and thus resulted in a stronger contractile force. In addition, cardiomyoctyes isolated from ouabain-treated EBs also appeared to have a larger internal store of calcium as evidenced by larger amplitude of caffeine-mediated calcium release. These changes could be related to the corresponding upregulation of key calcium handling proteins in cardiomyocytes isolated from ouabain-treated EBs. Specifically, the upregulated ryanodine receptor could result in the faster rate of calcium release, while the rate of calcium transient decay corresponds to the higher expression of SERCA in ouabain-treated cardiomyocytes. The enhanced intracellular calcium concentration due to Na+/K+-ATP inhibition nonetheless remains another plausible mechanism for such improvement.
Our results shed new light on the potential use of a hypertrophic stimulus on adult cardiomyocytes to enhance cardiac differentiation and maturation of ESC-CMs in vitro. Ouabain-driven cardiac differentiation of mESC-CMs is mediated by activation of Erk1/2 in the hypertrophy pathway. The relationship of the pathway with calcium handling in the cells was not identified. Nonetheless, our findings broaden knowledge of the differentiation processes of cultured ESCs, and may also contribute to the future development of step-cell based therapy for heart disease.
Author contribution
This research was designed by Chung-wah SIU, Hung-fat TSE, Chu-pak LAU, Deborah K LIEU, Cornilia MAN. The experiments were performed by Yee-ki LEE, Kwong-man NG, Wing-hon LAI. The new analytical tools and reagents were provided by Chung-wah SIU and Hung-fat TSE. Yee-ki LEE and Kwong-man NG were responsible for analyzing data. The manuscript was written by Yee-ki LEE, Kwong-man NG, and Chung-wah SIU.
References
Mummery CL, Ward D, Passier R . Differentiation of human embryonic stem cells to cardiomyocytes by coculture with endoderm in serum-free medium. Curr Protoc Stem Cell Biol 2007; Chapter 1: Unit 1F 2.
Siu CW, Moore JC, Li RA . Human embryonic stem cell-derived cardiomyocytes for heart therapies. Cardiovasc Hematol Disord Drug Targets 2007; 7: 145–52.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–7.
Moore JC, van Laake LW, Braam SR, Xue T, Tsang SY, Ward D, et al. Human embryonic stem cells: genetic manipulation on the way to cardiac cell therapies. Reprod Toxicol 2005; 20: 377–91.
Otsu K, Kuruma A, Yanagida E, Shoji S, Inoue T, Hirayama Y, et al. Na+/K+ ATPase and its functional coupling with Na+/Ca2+ exchanger in mouse embryonic stem cells during differentiation into cardiomyocytes. Cell Calcium 2005; 37: 137–51.
Passier R, Oostwaard DW, Snapper J, Kloots J, Hassink RJ, Kuijk E, et al. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells 2005; 23: 772–80.
Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 2003; 107: 2733–40.
Kim HS, Cho JW, Hidaka K, Morisaki T . Activation of MEK-ERK by heregulin-beta1 promotes the development of cardiomyocytes derived from ES cells. Biochem Biophys Res Commun 2007; 361: 732–8.
Braunwald E . Effects of digitalis on the normal and the failing heart. J Am Coll Cardiol 1985; 5: 51A–59A.
Schwartz A, Grupp G, Wallick E, Grupp IL . Ball WJ Jr . Role of the Na+K+-ATPase in the cardiotonic action of cardiac glycosides. Prog Clin Biol Res 1988; 268B: 321–38.
Akera T, Ng YC . Digitalis sensitivity of Na+, K+-ATPase, myocytes and the heart. Life Sci 1991; 48: 97–106.
Manunta P, Stella P, Rivera R, Ciurlino D, Cusi D, Ferrandi M, et al. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension 1999; 34: 450–6.
Balzan S, Neglia D, Ghione S, DUrso G, Baldacchino MC, Montali U, et al. Increased circulating levels of ouabain-like factor in patients with asymptomatic left ventricular dysfunction. Eur J Heart Fail 2001; 3: 165–71.
Gottlieb SS, Rogowski AC, Weinberg M, Krichten CM, Hamilton BP, Hamlyn JM . Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation 1992; 86: 420–5.
DUrso G, Frascarelli S, Balzan S, Zucchi R, Montali U . Production of ouabain-like factor in normal and ischemic rat heart. J Cardiovasc Pharmacol 2004; 43: 657–62.
DUrso G, Frascarelli S, Zucchi R, Biver T, Montali U . Cardioprotection by ouabain and digoxin in perfused rat hearts. J Cardiovasc Pharmacol 2008; 52: 333–7.
Huang L, Li H, Xie Z . Ouabain-induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J Mol Cell Cardiol 1997; 29: 429–37.
Haas M, Askari A, Xie Z . Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem 2000; 275: 27832–7.
Liu L, Zhao X, Pierre SV, Askari A . Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol 2007; 293: C1489–97.
Tian J, Liu J, Garlid KD, Shapiro JI, Xie Z . Involvement of mitogen-activated protein kinases and reactive oxygen species in the inotropic action of ouabain on cardiac myocytes. A potential role for mitochondrial KATP channels. Mol Cell Biochem 2003; 242: 181–7.
Au KW, Liao SY, Lee YK, Lai WH, Ng KM, Chan YC, et al. Effects of iron oxide nanoparticles on cardiac differentiation of embryonic stem cells. Biochem Biophys Res Commun 2009; 379: 898–903.
Mosmann T . Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63.
Hescheler J, Fleischmann BK, Lentini S, Maltsev VA, Rohwedel J, Wobus AM, et al. Embryonic stem cells: a model to study structural and functional properties in cardiomyogenesis. Cardiovasc Res 1997; 36: 149–62.
Peng M, Huang L, Xie Z, Huang WH, Askari A . Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J Biol Chem 1996; 271: 10372–8.
Umbhauer M, Marshall CJ, Mason CS, Old RW, Smith JC . Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature 1995; 376: 58–62.
Yao Y, Li W, Wu J, Germann UA, Su MS, Kuida K, et al. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci USA 2003; 100: 12759–64.
Eriksson M, Leppa S . Mitogen-activated protein kinases and activator protein 1 are required for proliferation and cardiomyocyte differentiation of P19 embryonal carcinoma cells. J Biol Chem 2002; 277: 15992–6001.
Davidson SM, Morange M . Hsp25 and the p38 MAPK pathway are involved in differentiation of cardiomyocytes. Dev Biol 2000; 218: 146–60.
Ding L, Liang XG, Hu Y, Zhu DY, Lou YJ . Involvement of p38MAPK and reactive oxygen species in icariin-induced cardiomyocyte differentiation of murine embryonic stem cells in vitro. Stem Cells Dev 2008; 17: 751–60.
Sauer H, Neukirchen W, Rahimi G, Grunheck F, Hescheler J, Wartenberg M . Involvement of reactive oxygen species in cardiotrophin-1-induced proliferation of cardiomyocytes differentiated from murine embryonic stem cells. Exp Cell Res 2004; 294: 313–24.
Chen Y, Amende I, Hampton TG, Yang Y, Ke Q, Min JY, et al. Vascular endothelial growth factor promotes cardiomyocyte differentiation of embryonic stem cells. Am J Physiol Heart Circ Physiol 2006; 291: H1653–8.
Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, et al. Absence of transverse tubules contributes to non-uniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev 2009; 18: 1493–500.
Liu J, Fu JD, Siu CW, Li RA . Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells 2007; 25: 303844.
Liu J, Lieu DK, Siu CW, Fu JD, Li R . Facilitated maturation of Ca2+ handling properties of human embryonic stem cell-derived cardiomyocytes by calsequestrin expression. Am J Physiol Cell Physiol 2009; 297: C152–9.
Bers DM . Cardiac excitation-contraction coupling. Nature 2002; 415: 198–205.
Satin J, Itzhaki I, Rapoport S, Schroder EA, Izu L, Arbel G, et al. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells 2008; 26: 1961–72.
Fu JD, Yu HM, Wang R, Liang J, Yang HT . Developmental regulation of intracellular calcium transients during cardiomyocyte differentiation of mouse embryonic stem cells. Acta Pharmacol Sin 2006; 27: 901–10.
Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 2008; 118: 507–17.
Wobu A, Guan K, Yang H, Boheler K . Embryonic stem cells as a model to study cardiac, skeletal muscle, and vascular smooth muscle cell differentiation. Methods Mol Biol 2002; 185: 127–56.
Acknowledgements
The project was supported by th grant HK RGC 777910 to Dr Chung-wah SIU and Prof Hung-fat TSE. We appreciated the help of Nil.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lee, YK., Ng, KM., Lai, WH. et al. Ouabain facilitates cardiac differentiation of mouse embryonic stem cells through ERK1/2 pathway. Acta Pharmacol Sin 32, 52–61 (2011). https://doi.org/10.1038/aps.2010.188
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.188
Keywords
This article is cited by
-
Epigallocatechin-3-gallate inhibits proliferation and migration of human colon cancer SW620 cells in vitro
Acta Pharmacologica Sinica (2012)
-
Calcium Homeostasis in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes
Stem Cell Reviews and Reports (2011)