Abstract
Aim:
To evaluate the effects of angiopoietin-1 (Ang-1) on myocardial endothelial cell function under high glucose (HG) condition.
Methods:
Mouse heart myocardial endothelial cells (MHMECs) were cultured and incubated under HG (25 mmol/L) or normal glucose (NG, 5 mmol/L) conditions for 72 h. MTT was used to determine cellular viability, and TUNEL assay and caspase-3 enzyme linked immunosorbent assays were used to assay endothelial apoptosis induced by serum starvation. Immunoprecipitation and Western blot analysis were used to analyze protein phosphorylation and expression. Endothelial tube formation was used as an in vitro assay for angiogenesis.
Results:
Exposure of MHMECs to HG resulted in dramatic decreases in phosphorylation of the Tie-2 receptor and its downstream signaling partners, Akt/eNOS, compared to that under NG conditions. Ang-1 (250 ng/mL) increased Tie-2 activation, inhibited cell apoptosis, and promoted angiogenesis. Ang-1-mediated protection of endothelial function was blunted by Ang-2 (25 ng/mL).
Conclusion:
Ang-1 activates the Tie-2 pathway and restores hyperglycemia-induced myocardial microvascular endothelial dysfunction. This suggests a protective role of Ang-1 in the ischemic myocardium, particularly in hearts affected by hyperglycemia or diabetes.
Similar content being viewed by others
Introduction
Angiopoietin-1 (Ang-1) has been shown to protect the heart against acute myocardial infarction under hyperglycemic conditions1. Hyperglycemic exacerbation of ischemic myocardial injury is strongly associated with high glucose-induced endothelial dysfunction2. Studies have also shown that Ang-1 has a protective effect on endothelial cells injured by hypoxia3, arsenite toxicity4, 5. However, few published data are available on the effects of Ang-1 on cardiac microvascular endothelium cultured under hyperglycemic conditions.
Our previous study demonstrated that diabetes or hyperglycemia disrupts Ang-1/Tie-2 signaling and attenuates Ang-1-induced angiogenesis in mouse models1. Forced over-expression of Ang-1 shifts the ratio of Ang-2 to Ang-1 and conveys protection from ischemic myocardial injury normally exacerbated by hyperglycemia6, 7. However, the protective mechanism of Ang-1 in ischemic myocardium under hyperglycemic conditions remains unclear. Here we hypothesize that Ang-1 may restore angiopoietin/Tie-2 balance and protect myocardial endothelial cells under high glucose (HG) conditions. To test our hypothesis, we characterized the activation of the Tie-2 pathway in mouse heart myocardial endothelial cells (MHMECs) under HG conditions. We also examined apoptosis and angiogenic responses to Ang-1 and Ang-2 stimulation. Our data indicate that Ang-1 increases Tie-2 activation and restores MHMEC function in HG conditions. Furthermore, increased Ang-2 attenuates the protective effects of Ang-1 under HG conditions.
Materials and methods
Materials
Endothelial cell growth medium (EGM-2) and fetal bovine serum (FBS) were purchased from Clonetics Co (MA, USA). The following primary antibodies were used: anti-phosphotyrosine (Upstate Co, NJ, USA), mouse anti-phospho-eNOS, mouse anti-eNOS (BD Co, CA, USA), rabbit anti-Ang-1 (Santa Cruz, CA, USA), rabbit anti-phospho-AKT, rabbit anti-Akt, mouse anti-cleaved caspase-3, mouse anti-Tie-2, and rabbit anti-β-actin (Cell Signaling, MA, USA). All reagents were of analysis grade.
Culture of MHMECs
Hearts were excised from 4-week-old mice (n=6) under aseptic conditions. Cardiac ventricles were cleaned by sterilized Hank's solution and then cut into pieces. A homogenate of ventricles was suspended and filtered by sequential 200-μm and 60-μm microfiltration. The filtered cells were washed twice with EGM-2, moved to a dish, and cultured in EGM-2 supplemented with 10% FBS, 2 mmol/L glutamine, 50 U penicillin G, and 0.05 mg/mL streptomycin at 37 °C in a humidified 95% air/5% CO2 incubator. Each of the endothelial lines used in these experiments had a typical cobblestone morphology, showed uptake of acetylated low-density lipoprotein, and exhibited factor VIII-related antigen. Primary cultures of MHMECs between passages 4 and 10 were used in all experiments.
Cell viability assay
MHMECs were cultured with either HG (25 mmol/L) or NG (5 mmol/L) media for 72 h and then transferred to 96-well plates with 1×105 cells/mL per well. MHMECs attached to the plates after 6-8 h and were starved in serum-free media for 48 h in the presence or absence of Ang-1 (250 ng/mL, R&D Systems, Inc, CA, USA). After treatment, MHMECs were washed twice with PBS, and MTT (final concentration of 500 mg/mL) was added to each well. MHMECs were incubated at 37 °C for 4 h, and then 100 mL of DMSO was added to dissolve the formed crystals. The absorbance measured at 570 nm was used to calculate the relative cell viability ratio.
Western blot analysis
MHMECs were washed with PBS, and 0.5 mL of TME lysis buffer [10 mmol/L Tris (pH 7.5), 5 mmol/L MgCl2, 1 mmol/L EDTA, and 25 mmol/L NaF] containing fresh 100 μmol/L Na3VO4, 20 μg/mL leupeptin, 1 μg/mL pepstatin A, 4 μg/mL aprotinin, and 1 mmol/L DTT was added. Cell lysates were prepared by freezing, thawing on ice, scraping, sonicating for 30 s, and centrifuging for 30 min at 15 000×g. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit. For Western blot analysis, 20 μg of protein was subjected to SDS-PAGE under reducing conditions, and proteins were then transferred to a polyvinylidene difluoride membrane as described previously8. The membrane was blocked for 2 h with a commercial blocking buffer from Life Technologies, Inc. The blots were incubated for 1 h with primary antibodies (1:1000 dilution), followed by a 1-h incubation with a secondary horseradish peroxidase-conjugated antibody. The presence of target proteins was revealed by a chemiluminescent assay (Amersham-Pharmacia Biotech).
Immunoprecipitation for Tie-2 phosphorylation
Each sample consisting of 1000 μg protein was incubated with 10 μL anti-Tie-2 antibody for 6-8 h at 4 °C, followed by the addition of 20 μL of protein G-Sepharose. The samples were then incubated overnight at 4 °C and centrifuged for 5 min at 1000×g at 4 °C. Protein-antibody complexes were washed once with buffer solution [10 mmol/L Tris (pH 7.4), 0.25 mmol/L EDTA, and 0.1% SDS], suspended in 30 μL loading buffer, heated for 5 min at 90-100 °C, and then centrifuged for 10 min at 12 000×g at 4 °C. The supernatant was subjected to immunoblot analysis. Tie-2 phosphorylation was revealed with an anti-phosphotyrosine antibody (1:1000).
Caspase-3 enzyme linked immunosorbent assay (ELISA)
MHMECs were cultured in either HG media or NG media for 72 h and then starved for 48 h to induce endothelial apoptosis in the presence or absence of Ang-1 or Ang-2 (R&D systems, Inc, CA, USA). Cell culture supernatants were collected and analyzed using commercial caspase-3 enzyme-linked immunosorbent assay kits (Sigma, MO, USA). The optical density of the wells was determined by spectrophotometry at a wavelength of 450 nm.
TUNEL assay
MHMECs were cultured with either HG or NG media for 72 h and then transferred to two-well chamber slides with a cell density of 2×105 cells/mL per well. MHMECs were attached to the slides after 6-8 h and were cultured in serum-free media for 48 h in the presence or absence of Ang-1 or Ang-2. Slides were fixed with 10% formaldehyde for 30 min. Cells were then submitted to terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) according to the manufacturer's instructions (Promega, CA, USA). The apoptotic cells were stained green. Nuclei were counterstained with DAPI in blue. Apoptosis was quantified by counting TUNEL-positive cells per 100 DAPI cells9.
Endothelial tubular formation assay
MHMECs were plated on 48-well (1×105 cells/well) chamber slides (BD Falcon). After 6–8 h, the cells were washed with appropriate serum-free media and overlaid with matrigel (Sigma, St Louis, MO, USA) diluted 1:1 in EGM-2 media supplemented with 10% FBS. After a 30-min gel formation at 37 °C in the incubator, the gels were overlaid with 0.5 mL of culture media. The culture medium was supplemented with Ang-1 or Ang-2 in either HG or NG media. The cells were incubated for 24 h at 37 °C for full development of capillary-like network structures. The gels were photographed using a phase-contrast microscope. Endothelial tube formation was quantified by counting the number and cumulative length of tubular structures in six fields of each well with image acquisition and analysis software (Image Pro-express software, CA, USA). Each assay was performed in duplicate.
Adenovirus transfection
Ad-Ang-1 encodes mouse Ang-1. Adenoviruses (Adenovirus Co, CA, USA) were amplified using a human embryonic kidney cell line (HEK 293) as a host. The titers of the lysates were 1×109 pfu/mL for Ad-Ang-1 and Ad-β-gal (adenovirus-β-galactosidase). MHMECs were transfected with 20 pfu/cell of Ad-Ang-1 or Ad-β-gal in serum-free medium for 24 h. Then the infection medium was removed, and cells were incubated with HG or NG media for 72 h.
Statistical analysis
The values are expressed as the mean±SE. Statistical analysis of the data was performed using Student's t-tests and ANOVA where appropriate. Values of P<0.05 were considered statistically significant.
Results
Ang-1-induced activation of Tie-2 is impaired by high glucose
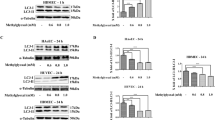
We used immunoprecipitation to assay the activation of Tie-2. A Tie-2 antibody was used to precipitate the protein, and a phospho-tyrosine antibody was used to blot and assay the activation of Tie-2 because phosphorylated Tie-2 antibodies were unavailable. Exposure of MHMECs to HG for 72 h dramatically decreased the activation of Tie-2 receptors compared that under NG conditions (Figure 1A). Furthermore, HG significantly inhibited Akt/eNOS phosphorylation (Figure 1B), the major downstream event of the Ang-1/Tie-2 signaling pathway. Previously, we showed that Ang-1 activates Ang-1/Tie-2/Akt signaling, which is blunted by hyperglycemia in diabetic mouse models1. After MHMECs were incubated under HG or NG conditions for 72 h, we treated MHMECs with 250 ng/mL Ang-1 for 15 min. Consistent with our previous findings6, our data show that Ang-1 significantly increased Tie-2 phosphorylation under both NG and HG conditions. Furthermore, our present study reveals that Ang-1-induced Tie-2 phosphorylation is attenuated in HG conditions compared to NG conditions (Figure 1C).
Changes in Tie-2/Akt/eNOS signaling pathways under HG conditions. (A) Tie-2 phosphorylation was decreased under HG conditions in MHMECs (n=3). (B) HG inhibited phosphorylation of Akt and eNOS in MHMECs compared with NG groups (n=5). (C) Ang-1 increased Tie-2 phosphorylation in MHMECs, which was dampened under HG conditions (n=3). bP<0.05 compared with NG groups.
Ang-1 attenuated HG-induced endothelial cell dysfunction in MHMECs
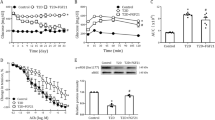
After MHMECs were incubated under either HG or LG conditions for 72 h, we treated the MHMECs with serum-free media in the presence or absence of 250 ng/mL Ang-1 for 48 h. We then examined the effects of Ang-1 on apoptosis of MHMECs using MTT, caspase-3, and TUNEL assays. Our MTT results show that Ang-1 increased cellular viability from 88.56%±3.90% to 112.43%±9.28% under HG conditions (Figure 2A). This was accompanied by a significant suppression of caspase-3 activation under HG conditions (Figure 2B). Serum starvation-induced apoptosis was revealed by green staining in the TUNEL assay and was observed in a significantly higher proportion of non-Ang-1-treated cells (P<0.01) compared to cells treated with Ang-1 (14.03%±3.44% vs 6.25%±0.48%, respectively, P<0.05) under HG conditions (Figure 2C).
Ang-1 inhibited myocardial endothelial apoptosis induced by HG. (A) Ang-1 increased cellular viability under HG conditions, as observed by MTT assays. (B) Representative Western blot and densitometry analysis of caspase-3 activation. (C) Representative images of a TUNEL assay showing that Ang-1 significantly decreased MHMEC apoptosis induced by HG (n=3, 400× magnification). bP<0.05, cP<0.01.
Angiogenesis is another important function of endothelial cells10. Our data clearly show that Ang-1 significantly promoted myocardial endothelial tubular formation that was significantly impaired by HG conditions (Figure 3).
Ang-2 exacerbated MHMEC dysfunction induced by HG
Ang-2, which is increased in diabetic patients, is a natural antagonist of Ang-1. However, few published data are available on the effects of Ang-2 on endothelial cells under HG conditions. After MHMECs were incubated under HG or NG conditions for 72 h, we treated the MHMECs continuously with serum-free media in the presence or absence of Ang-2 (25 ng/mL) for 48 h. We observed that increased Ang-2 exacerbated the endothelial apoptosis induced by HG (Figure 4). When we used Ang-2 siRNA to knock down Ang-2 protein expression, MHMEC apoptosis was decreased significantly under HG conditions (Figure 5).
RNA interference of Ang-2 inhibited endothelial cell apoptosis induced by HG. (A) Western blot analysis shows that the expression of Ang-2 was significantly inhibited by RNA interference in endothelial cells 24 h after the transfer of Ang-2 siRNA. (B) Quantification of endothelial apoptosis by TUNEL staining. (C) Caspase-3 activation was assayed by ELISA (n=3). bP<0.05.
Ang-1-mediated protection of MHMEC function is impaired by Ang-2 under HG conditions
MHMECs were incubated in HG media for 72 h and then sequentially supplemented with both Ang-2 (25 ng/mL) and Ang-1 (250 ng/mL) or Ang-1 alone6. MHMEC apoptosis and tubular formation were analyzed by the methods listed above. Exposure of MHMECs to Ang-2 led to increased activation of caspase-3 and an increased apoptotic ratio in HG conditions, whereas Ang-1 suppressed these effects (Figure 6A, 6B). Intriguingly, increased Ang-2 significantly delayed Ang-1-induced tubular formation in MHMECs under HG conditions (Figure 6C).
MHMECs do not express detectable Ang-11 and were thus transfected with Ad-Ang-1 to over-express Ang-1 (Figure 7A). The transfected MHMECs were incubated with HG or NG media for 72 h and then treated with serum-free media for 48 h in the presence or absence of Ang-2. The activation of caspase-3 in all Ad-Ang-1 groups was lower than that of Ad-β-gal groups in both HG and LG conditions. However, Ang-2 increased the activation of caspase-3 (Figure 7B).
Ang-2 increased the activation of caspase-3 inhibited by Ang-1 in MHMECs. (A) Western blot analysis shows that the expression of Ang-1 was significantly increased in endothelial cells transfected with Ad-Ang-1 after 24 h. Control: untransfected cells; Ad-β-gal: cells were transfected with control adenovirus vector; Ad-Ang-1: cells were transfected with an adenovirus vector encoding mouse Ang-1. (B) Ang-2 increased caspase-3 activation, as assayed by ELISA (n=3). bP<0.05 compared with the Ad-β-gal groups; eP<0.05 compared with the Ad-Ang-1 groups.
Discussion
The risk of morbidity and mortality associated with cardiovascular disease is significantly increased in patients who are diabetic compared to patients who are non-diabetic11. This increase in risk may be related to vascular endothelial cell dysfunction that occurs under diabetic and hyperglycemic conditions. The angiopoietin system plays a critical role in maintaining vascular integrity and endothelial function in diabetic patients12, 13. Angiopoietins comprised of the ligands Ang-1/-2/-3/-4 represent a new family of angiogenic factors. Ang-1 and Ang-2 are the two key ligands for the Tie-2 receptor14. Tie-2 is a receptor tyrosine kinase that is expressed in the vascular endothelium. Ang-1 binds to the Tie-2 receptor and induces Tie-2 phosphorylation, thus promoting endothelial cell survival and angiogenesis by activating the PI3 kinase-Akt-eNOS pathway15. Therefore, we examined the Tie-2 signaling pathway under HG conditions. Our immunoprecipitation and Western blot data show that exposure of MHMECs to HG resulted in a dramatic decrease in phosphorylation of the Tie-2/Akt/eNOS signaling pathway compared to MHMECs exposed to NG conditions.
Because Ang-1-mediated protection of endothelial cells involves the Ang-1/Tie-2/Akt pathways15, 16, 17, we observed the effects of Ang-1 on MHMECs under HG conditions. Our data show that Ang-1 significantly increased Tie-2 phosphorylation, which was inhibited by HG, and suppressed serum starvation-induced caspase-3 activation and endothelial cell apoptosis under HG conditions. Our data suggest that Ang-1 inhibits microvascular endothelial apoptosis, which may be responsible for the protection of Ang-1 in the ischemic heart, particularly in a hyperglycemic environment.
Angiogenesis is an important function of the cardiac microvascular endothelium, which is impaired in diabetes10. Capillary tube formation, which depends on endothelial migration and proliferation18, 19, is the primary mechanism of angiogenesis. In the present study, we also investigated the effect of Ang-1 on angiogenesis in the cardiac microvascular endothelium with tubular formation in vitro. Our data reveal that Ang-1 promoted myocardial endothelial tubular formation that was dampened by hyperglycemia. These data further suggest that Ang-1 can increase angiogenesis, benefiting ischemic hearts, particularly those in a hyperglycemic environment.
Ang-2 has been identified as a natural antagonist of Ang-1, inhibiting Ang-1-mediated Tie-2 phosphorylation in the presence of Ang-11, 6, 20, 21. Our previous studies have shown that exposure of MHMECs to HG led to a significant increase in Ang-2 expression and a decrease in Tie-2 expression compared to NG conditions6. However, the functional roles of Ang-2 on myocardial endothelial cells under HG conditions were not explored. In the present study, we found that increased Ang-2 strikingly exacerbated myocardial endothelial apoptosis and delayed tubular structure formation of MHMECs under HG conditions. The endothelial protection mediated by Ang-1 was diminished in the presence of increased Ang-2 under HG conditions, which is consistent with our in vivo data1, 6. Additionally, our data suggest that Ang-1 shifts the ratio of Ang-2 to Ang-1 and protects myocardial endothelial cells under hyperglycemic conditions.
In the present study, we have demonstrated that Ang-1 can inhibit microvascular endothelial apoptosis and increase angiogenesis under HG conditions, possibly playing a role in protecting ischemic hearts in hyperglycemic or diabetic individuals.
Author contribution
Jian-xiong CHEN and Duan-fang LIAO designed the studies; Qin-hui TUO conducted the experiments and wrote the paper. Drs Guo-zuo XIONG, Heng ZENG, and Hei-di YU performed MHMEC isolation, cell culture, and immunoprecipitation; Hong-yan LING, Bing-yang ZHU, and Shao-wei SUN assisted with data analysis.
References
Tuo QH, Zeng H, Stinnett A, Yu H, Aschner JL, Liao DF, et al. Critical role of angiopoietins/Tie-2 in hyperglycemic exacerbation of myocardial infarction and impaired angiogenesis. Am J Physiol Heart Circ Physiol 2007; 292: H1664–74.
Farhangkhoee H, Khan ZA, Kaur H, Xin X, Chen S, Chakrabarti S . Vascular endothelial dysfunction in diabetic cardiomyopathy: pathogenesis and potential treatment targets. Pharmacol Ther 2006; 111: 384–99.
Wang YL, Hui YN, Guo B, Ma JX . Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye (Lond) 2007; 21: 1501–10.
Kugathasan L, Ray JB, Deng Y, Rezaei E, Dumont DJ, Stewart DJ . The angiopietin-1-Tie2 pathway prevents rather than promotes pulmonary arterial hypertension in transgenic mice. J Exp Med 2009; 206: 2221–34.
Park JS, Seo J, Kim YO, Lee HS, Jo I . Coordinated regulation of angiopoietin-1 and vascular endothelial growth factor by arsenite in human brain microvascular pericytes: implications of arsenite-induced vascular dysfunction. Toxicology 2009; 264: 26–31.
Chen JX, Stinnett A . Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol 2008; 28: 1606–13.
Tuo QH, Liao DF . The ratio of Ang-1/Ang-2 in vessel diseases. Chin J Arterioscler 2009; 17: 414–6.
QH Tuo, C Wang, FX Yan, DF Liao . MAPK pathway mediates the protective effects of onychin on oxidative stress-induced apoptosis in ECV304 endothelial cells. Life Sci 2004; 76: 487–97.
Tuo QH, Liang L, Zhu BY, Cao X, Liao DF . The effect of Daxx on the cholesterol accumulation in hepatic cells. World J Gastroenterol 2008; 14: 435–40.
Lovren F, Pan Y, Shukla PC, Quan A, Teoh H, Szmitko PE, et al. Visfatin activates eNOS via Akt and MAP kinases and improves endothelial cell function and angiogenesis in vitro and in vivo: translational implications for atherosclerosis. Am J Physiol Endocrinol Metab 2009; 296: E1440–9.
Coutinho M, Gerstein HC, Wang Y, Yusuf S . The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999; 22: 233–40.
Singh H, Brindle NP, Zammit VA . High glucose and elevated fatty acids suppress signaling by the endothelium protective ligand angiopoietin-1. Microvasc Res 2010; 79: 121–7.
Minhas N, Xue M, Fukudome K, Jackson CJ . Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J 2010; 24: 873–81.
Gomei Y, Nakamura Y, Yoshihara H, Hosokawa K, Iwasaki H, Suda T, et al. Functional differences between two Tie2 ligands, angiopoietin-1 and -2, in regulation of adult bone marrow hematopoietic stem cells. Exp Hematol 2010; 38: 82–9.
Fujikawa K, de Aos Scherpenseel I, Jain SK, Presman E, Christensen RA, Varticovski L . Role of PI 3-kinase in angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells. Exp Cell Res 1999; 253: 663–72.
Huang J, Bae JO, Tsai JP, Kadenhe-Chiweshe A, Papa J, Lee A, et al. Angiopoietin-1/Tie-2 activation contributes to vascular survival and tumor growth during VEGF blockade. Int J Oncol 2009; 34: 79–87.
Chen JX, Lawrence ML, Cunningham G, Christman BW, Meyrick B . HSP90 and Akt modulate Ang-1-induced angiogenesis via NO in coronary artery endothelium. J Appl Physiol 2004; 96: 612–20.
Risau W . Mechanisms of angiogenesis. Nature 1997; 386: 671–4.
Kern J, Bauer M, Rychli K, Wojta J, Ritsch A, Gastl G, et al. Alternative splicing of vasohibin-1 generates an inhibitor of endothelial cell proliferation, migration, and capillary tube formation. Arterioscler Thromb Vasc Biol 2008; 28: 478–84.
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277: 55–60.
Yang M, Zhang M, Chen J, Mukherjee R, Zhang L, Lin S, et al. Angiopoietin-1 inhibits mouse glomerular endothelial cell senescence via Tie2 receptor-modulated ERK1/2 signaling. Am J Nephrol 2010; 31: 490–500.
Acknowledgements
This work was supported by grants from the American Heart Association (0565196B) and the NIH (DK074995 and R01HL102042), the National Natural Science Foundation of China (No 30971170, 30770868, 30971267, and 30600249), and the Outstanding Youth Foundation of Education Department in Hunan Province (09B089).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tuo, Qh., Xiong, Gz., Zeng, H. et al. Angiopoietin-1 protects myocardial endothelial cell function blunted by angiopoietin-2 and high glucose condition. Acta Pharmacol Sin 32, 45–51 (2011). https://doi.org/10.1038/aps.2010.183
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.183
Keywords
This article is cited by
-
Angiopoietin-1 and Angiopoietin-2 in metabolic disorders: therapeutic strategies to restore the highs and lows of angiogenesis in diabetes
Journal of Endocrinological Investigation (2016)
-
Fetuin-A and angiopoietins in obesity and type 2 diabetes mellitus
Endocrine (2012)