Abstract
Aim:
JG3, a novel marine-derived oligosaccharide, significantly inhibits angiogenesis and tumor metastasis by blocking heparanase activity. It also arrests tumor growth, an effect that is not fully explained by its anti-heparanase activity. Here we sought to identify the mechanisms underlying JG3-mediated inhibition of tumor growth.
Methods:
Heparanase expression was assessed by RT-PCR and Western blotting. NF-κB activation status was determined using immunofluorescence, Western blotting, DNA-binding and transcription-activity assays. The effect of JG3 on upstream components of the NF-κB pathway and on selected transcription factors were monitored by Western blotting. The antitumor effect of JG3 and its relation to NF-κB activation were evaluated using four different tumor xenograft models.
Results:
We found that JG3 effectively inhibited NF-κB activation independent of heparanase expression. Our results indicate that JG3 inactivated NF-κB by interfering with the activation of upstream components of the NF-κB pathway without generally affecting the nuclear translocation of transcription factors. Further, in vivo studies demonstrated that JG3 effectively arrested the growth of tumors derived from cell lines in which NF-κB was constitutively active (BEL-7402 liver carcinoma and MDA-MB-435s breast carcinoma), but did not affect the growth of tumors derived from NF-κB-negative cell lines (SGC-7901 gastric cancer and HO-8910 ovarian carcinoma).
Conclusion:
Our data indicate that NF-κB mediates the JG3-induced arrest of tumor growth. These results define a new mechanism of action of JG3 and highlight the potential for JG3 as a promising lead molecule in cancer therapy.
Similar content being viewed by others
Introduction
Previous results from our group revealed that JG3 (Supplementary Figure 1A), a novel marine-derived oligosaccharide, significantly inhibits lung metastasis in a murine B16F10 experimental metastasis model, as well as angiogenesis and lung metastasis of MDA-MB-435s orthotopic xenografts in athymic mice. These effects were mediated by inhibition of heparanase activity via binding of JG3 to the KKDC and QPLK domains within heparanase1.
Heparanase is an endoglycosidase that cleaves heparan sulfate. Because heparan sulfate contributes significantly to the integrity of extracellular matrix (ECM) and to cell-ECM interactions, cleavage of heparan sulfate is expected to play an important role in cell adhesion, metastatic and angiogenic processes2, 3, 4, 5. There is also clinical evidence that over-expression of heparanase in cancers, such as gastric cancer, gallbladder cancer and pancreatic adenocarcinoma, is associated with decreased post-operative survival and frequent metastasis. Additionally, high levels of heparanase expression have been shown to correlate well with nuclear factor kappa B (NF-κB)activation6, 7, 8.
NF-κB is a ubiquitously expressed family of transcription factors known to play a role in a wide spectrum of cellular functions including cell cycle regulation, apoptosis, migration and angiogenesis; they are also involved in immune and inflammatory responses9, 10, 11, 12. NF-κB activation is under strict control. Under basal conditions, NF-κB is sequestrated in the cytoplasm by the inhibitor of κB (IκB). Upon activation, IκB kinase (IKK) phosphorylates IκB promoting its ubiquitin-dependent degradation. Liberated NF-κB then migrates into the nucleus and induces transcription of target genes10, 13.
In our previous study, JG3 was also shown to arrest tumor growth, an action that was difficult to fully explain based on heparanase mediated effects. Here, we extend these previous studies, seeking to identify the mechanisms underlying the tumor growth inhibitory effect of JG3.
Materials and methods
Materials
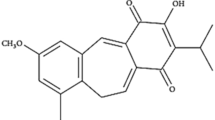
JG3 was obtained by semi-synthesis following sulfate modification by reacting oligomannurarate with ClSO3H in formamide. The pH of products was adjusted to 7.0 with 4 mol/L NaOH, and desalted using Sephadex G-10. The product peaks were pooled and freeze-dried. The molecular weights of JG3 were analyzed by High Performance Gel Permeation Chromatography (HPGPC) using a G3000PWxl column (300 mm×7.8 mm) (TOSOH, Japan)14. The structure of JG3 is depicted in Supplemental Figure 1A.
Western blot analysis
Whole-cell protein lysates and cytoplasmic or nuclear extracts were separated by SDS-PAGE, electroblotted onto nitrocellulose membranes and probed with antibodies against p-IKK (S176/180), p-IκB(S32), IκB, RhoA and Bcl-2 (1:1000 dilution, Cell signaling, Beverly, MA, USA), as well as p65 (RelA), heparanase, COX2 and GAPDH (1:1000 dilution; Santa Cruz Biotechnology, Inc CA, USA).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
MDA-MB-435s cells were pretreated with 100 μg/mL JG3 for different times and then total RNA was isolated. Total RNA (2.5 μg) was reverse transcribed into cDNA by incubating at 50 °C for 60 min. Heparanase mRNA was amplified from cDNA templates by RT-PCR using the primers 5′-CCG CTC GAG ATG CTG CTG CGC-3′ (sense) and 5′-CCG GAA TTC TCA GAT GCA AGC AGC A-3′ (antisense); human GAPDH was amplified as an internal control using purchased primers (Sangon, Shanghai China). PCR cycling conditions were as follows: 94 °C for 5 min (initial denaturation), followed by 35 cycles of 94 °C for 45 s, 60 °C for 45 s and 72 °C for 1 min followed by a final extension step of 72 °C for 10 min. Products were electrophoresed on 2% agarose gels, stained with ethidium bromide and visualized under UV light.
Electrophoretic mobility shift assays
BEL-7402 cells were pretreated with 100 μg/mL JG3 for different times or left untreated. The nuclear extracts were prepared as described previously15. Supernatant protein concentrations were quantified using the BCA protein assay kit. Electrophoretic mobility shift assay (EMSA) was performed using the LightShift Chemiluminescent EMSA kit (Pierce Inc, Rockford, IL, USA) with an NF-κB biotin-labeled oligonucleotide.
NF-κB luciferase reporter assays
NF-κB luciferase reporter assays were performed as described previously16. Briefly, cells were co-transfected with an NF-κB responsive 3×κBL luciferase reporter construct and a renilla luciferase-expressing plasmid (internal control to normalize for transfection efficiency) using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen CA, USA). After treatment for different times, firefly and renilla luciferase activities were assessed using a dual luciferase reporter gene assay kit (Beyotime, China). NF-κB transcriptional activity=(relative light units of firefly luciferase/relative light units of renilla luciferase)×100.
Antitumor activity against human tumor xenografts in nude mice
Under sterile conditions, well-developed BEL-7402 liver tumors, SGC-7901 gastric tumors or HO-8910 ovarian carcinomas were cut into 1-mm3 fragments and transplanted subcutaneously (sc) into the right flank of recipient nude mice. For the breast cancer model, 5×106 MDA-MB-435s cells was implanted into the mammary fat pads. When tumor volumes reached 100 mm3, the mice were randomly assigned into control and treatment groups. The control group was given normal saline, and treatment groups received different dose of JG3 (5, 20 or 40 mg/kg). JG3 was given via intravenously (iv) three times weekly or via daily sc injection. The sizes of tumors and the body weights of mice in each group were measured twice per week. Tumor volume=(length×width2)/2. The individual relative tumor volume (RTV) was calculated as Vt/V0, where Vt is the volume on each day, and V0 is the volume at the beginning of the treatment.
Immunohistochemistry
Tumor samples were fixed overnight in 10% neutral-buffered formalin and embedded in paraffin. Immunohistochemistry experiments were performed using a rabbit polyclonal anti-p65 antibody (Boster, Wuhan, China), diluted 1:50, and biotin-labeled rabbit IgG (Santa Cruz Biotechnology, Inc, CA, USA).
Statistical analysis
Data were presented as means±SEM, and differences were considered significant when P<0.05 as determined by Student's t-tests.
Results
JG3 blocks NF-κB activation independently of heparanase status
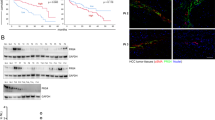
Our previous results showed that JG3 short term treatment (2 h) could effectively inhibit heparanase activity1. To determine whether JG3 also influenced heparanase expression, we first performed Western blot assays to assess heparanase protein expression. In MDA-MB-435s cells, which over-express heparanase, treatment with 100 μg/mL JG3 for 24 h markedly reduced heparanase protein expression. RT-PCR assays confirmed these results, showing that heparanase mRNA level was decreased after JG3 treatment (Figure 1A). These observations indicate that long-term treatment with JG3 acted at the transcriptional level to reduce heparanase expression.
JG3 blocks NF-κB activation independently of heparanase status. (A) JG3 inhibits heparanase expression. MDA-MB-435s cells were treated with 100 μg/mL JG3 for the indicated time, then heparanase mRNA (upper) and protein (lower) levels were analyzed by RT-PCR and Western blotting, respectively. GAPDH was used as an internal standard. (B and C) Immunofluorescence assays show that JG3 blocks nuclear translocation of p65 (green) in MDA-MB-435s cells (B) and BEL-7402 cells (C). Nuclei were counterstained with DAPI (blue). Scale bars, 15 μm.
NF-κB, which is well known to play important roles in tumor growth, angiogenesis and tumor metastasis, has also been reported to regulate heparanase expression6, 9. Based on this, we speculated that JG3 might act through inhibition of NF-κB activation to reduce heparanase expression. Accordingly, we next sought to determine whether JG3 inhibited NF-κB activation in MDA-MB-435s cells using RelA (p65), a central component of NF-κB, as a probe to monitor the activation status of NF-κB. We found that NF-κB was constitutively activated in intact MDA-MB-435s cells. After exposure to 100 μg/mL JG3 for 24 h, nuclear p65 levels were reduced, indicating inhibition of NF-κB activation (Figure 1B).
Because heparanase is overexpressed and NF-κB is highly activated in MDA-MB-435s cells, to test the role of heparanase in mediating JG3-induced inhibition of NF-κB, we extend our analysis to human liver cancer BEL-7402 cells, in which NF-κB is highly activated but heparanase expression is low1. As shown in Figure 1C, under the same regimen, 100 μg/mL JG3 also effectively reduced nuclear p65 levels in BEL-7402 cells, implying that JG3-induced inhibition of NF-κB activation is independent of heparanase expression level.
JG3 inhibits constitutive NF-κB activation
Because NF-κB activation involves several key steps, including NF-κB translocation into the nucleus, binding to the specific DNA sequence and regulating the transcription of target genes, we examined the NF-κB-inactivator function of JG3 from several perspectives.
We first tested the effect of JG3 on NF-κB nuclear translocation. Our results showed that exposure to 100 μg/mL JG3 for 24 h induced an increase in cytoplasmically sequestered p65 and a corresponding decrease in nuclear p65 compared with controls, indicating that JG3 inhibits NF-κB nuclear translocation. Importantly, JG3 did not influence total p65 protein expression (Figure 2A).
JG3 blocks constitutive NF-κB activation. (A) JG3 blocks p65 nuclear translocation. BEL-7402 cells were exposed to 100 μg/mL JG3 for the indicated time, and Western blot assays were used to determine the total amount of p65 protein and its distribution in the cytoplasm and nucleus. GAPDH and histone 3 were used as internal controls for cytoplasmic and nuclear proteins, respectively. (B) JG3 attenuates NF-κB DNA binding. BEL-7402 cells were treated with 100 μg/mL JG3 for 24 h and analyzed for gel-shift activity using a biotin-labeled NF-κB consensus oligonucleotide. (C) JG3 attenuates NF-κB transcriptional activity, assessed by reporter assay (P<0.05, n=9). BEL-7402 cells were co-transfected with pNF-luc and renilla constructs, and then treated with 100 μg/mL JG3 for the indicated time before measuring NF-κB-dependent luciferase activity. D) JG3 reduces expression of NF-κB target genes. BEL-7402 cells were treated with 100 μg/mL JG3 for the indicated time, then expression of target genes was evaluated by Western blotting using the indicated antibodies.
To further assess the functional consequences of this reduced NF-κB nuclear translocation, we next evaluated the effect of JG3 on the ability of NF-κB to bind to specific DNA sequences in target genes. Using EMSA assay, we found that treatment with 100 μg/mL JG3 for 24 h, decreased the DNA binding of NF-κB to approximately 30% of the control (Figure 2B).
Because NF-κB activation is ultimately embodied in a change in the transcription of target gene, we next used a reporter assay to confirm the inhibitory effect of JG3 on the transcriptional function of NF-κB. Figure 2C shows that 100 μg/mL JG3 induced a time-dependent suppression of NF-κB transcriptional activity, reducing the transcriptional activity of NF-κB at 36 h by about 25% compared with the control (P<0.05). Finally, we tested the effect of JG3 on selected NF-κB target genes that are important in metastasis. The results demonstrated that 100 μg/mL JG3 markedly inhibited RhoA, Bcl-2 and COX2 protein expression in a time-dependent manner.
Collectively, the results from multiple experimental approaches indicate that JG3 is capable of effectively blocking NF-κB activation.
JG3 inhibits NF-κB by interfering with upstream signaling
The above results demonstrated that JG3 significantly repressed p65 nuclear translocation and subsequent NF-κB-dependent transcriptional activity. Next, we examined whether JG3 also affected the nuclear translocation of other transcript factors. Using Western blot analysis of nuclear and cytoplasmic fractions, we tested the effect of JG3 on the sub-cellular location of three important transcription factors. As shown in Figure 3A, the sub-cellular location of these three transcription factors differed from one another, and were unaffected by treatment with 100 μg/mL JG3 for 24 h. These data indicate that JG3 did not have a general effect on the nuclear translocation of transcription factors, but instead specifically inhibited NF-κB nuclear translocation.
Effect of JG3 on NF-κB upstream signaling components and other transcription factors. (A) JG3 does not alter the subcellular distribution of other transcription factors. BEL-7402 cells were treated with 100 μg/mL JG3 for the indicated time, then the cellular distribution of some important transcription factors was tested by Western blotting using the indicated antibodies. GAPDH and histone 3 were used as internal controls for cytoplasmic and nuclear proteins, respectively. (B) JG3 inhibits IκB and IKK activation/phosphorylation. BEL-7402 cells were exposed to 100 μg/mL JG3 for the indicated time and assayed for IKK and IκB phosphorylation status and total amount of IκB by Western blotting. GAPDH was used as an internal standard.
To further explore the mechanism underlying JG3 inhibition of NF-κB activation, we focused on the direct upstream regulators, IκB and IKK. Exposure to 100 μg/mL JG3, particularly after 24 h caused a marked decrease in IKK and IκB phosphorylation accompanied by an increase in IκB levels (Figure 3B), suggesting that JG3 inactivates NF-κB by targeting upstream signaling pathway components.
JG3 arrests tumor growth by blocking NF-κB activation
The above results revealed that JG3 blocks NF-κB activation in vitro. Given the important role of NF-κB in cell proliferation, we speculated that NF-κB might be involved in the inhibitory effect of JG3 on tumor growth. To confirm this, we assessed the inhibitory effects of JG3 on the growth of tumors with different NF-κB activated statuses. We chose four tumor xenograft models: two with highly constitutive activated NF-κB (BEL-7402 human liver cancer and MDA-MB-435s human breast cancer) and two that were negative or lower for NF-κB activation (SGC-7901 human gastric cancer and HO-8910 ovarian epithelial carcinoma, Supplementary Figure 1B).
JG3 was effective against the growth of both tumors with constitutively active NF-κB. BEL-7402 xenograft growth was significantly inhibited by JG3 (40 mg/kg, daily for 14 d via subcutaneous route) which reduced tumor volume by 43.5% (P<0.05, Figure 4A). In parallel, MDA-MB-435s xenograft growth was suppressed by 57.3% by three injections (iv) per week of 20 mg/kg JG3 (P<0.01, Figure 4B). Notably, JG3 was well tolerated at the doses tested, and the animals showed no signs of toxicity or body weight loss over the course of the experiments (data not shown). In contrast, JG3 had no effect on the growth of SGC-7901 or HO-8910 xenografts at 40 mg/kg (sc) or 20 mg/kg (iv), respectively (Figure 4C and D). Taken together, these data indicate that the ability of JG3 to arrest tumor growth is closely correlated with the NF-κB-activation status of the tumors.
JG3 arrests tumor growth and antagonizes the activation of NF-κB in different tumors. (A and B) Effect of JG3 on the growth of liver carcinoma BEL-7402 and breast cancer MDA-MB-435s xenografts. (C and D) Effect of JG3 on the growth of ovary carcinoma HO-8910 and gastric cancer SGC-7901 xenografts. Tumor-bearing mice were treated with the regimen shown in the figure. (The BEL-7402 study was ended on day 14 because the tumor volume reached 2000 mm3). For sc injection, mice were given daily doses of 5 or 40 mg/kg. For iv injection, mice were given 5 or 20 mg/kg JG3 three times per week. Normal saline was used as a negative control. All data are expressed as means±SEMs of a typical experiment. Comparisons between treatment and control groups were made using Student's t-test (bP<0.05, cP<0.01, n=10). (E) JG3 antagonizes the activation of NF-κB in vivo. NF-κB activation status in different tumor tissue samples was assessed immunohistochemically by staining for p65 (brown) and nuclei (blue). Scale bars, 75 μm. The local area indicated by the arrowhead was further magnified and is shown in the corner.
We further tested NF-κB activation status in tumor samples during treatment using immunohistochemistry. Figure 4E shows that NF-κB was constitutively activated in BEL-7402 tumor tissue. In contrast, NF-κB was mainly sequestered in the cytoplasm of cells in HO-8910 tumor tissue from the negative control group. Notably, JG3 treatment decreased NF-κB activation in BEL-7402 tumor tissue, but had little influence in HO-8910 tumor tissue. Collectively, these observations underscore the importance of the NF-κB pathway in the inhibitory effect of JG3 on tumor growth.
Discussion
In the present study, we showed that JG3, a sulfated oligomannurarate, effectively arrested NF-κB activation both in vitro and in vivo. This is evidenced by the fact that JG3 blocked p65 nuclear translocation and inhibited subsequent DNA–NF-κB binding and NF-κB-dependent transcription, and by immunohistochemistry assays, which confirmed that JG3 administered sc markedly inhibited NF-κB activation in tumor tissues. Collectively, these data indicate that JG3 functions as a novel NF-κB inhibitor.
An in vivo antitumor study employing four types of tumors with different NF-κB activation status showed that JG3 exerted anti-growth effects only on the two tumor-types that possessed high constitutive NF-κB activity; those with inactive NF-κB were unaffected by JG3. The anti-NF-κB potency of JG3 was closely correlated with its efficacy in arresting tumor growth. Consistent with the observation that JG3-mediated inhibition of NF-κB activity was independent of its effects on heparanase expression. Western blot analyses showed no correlation between the expression of heparanase in these four tumor cell lines and the anti-growth effect of JG3 (data not shown). Collectively, these data indicate that the NF-κB pathway, rather than heparanase, is primarily involved in mediating the antitumor effects of JG3.
NF-κB is a ubiquitously expressed family of transcription factors that plays a vital role in tumor development and progression, reflecting their ability to control the expression of a number of genes involved in cell apoptosis, angiogenesis, tumor invasion and metastatic processes9. Several clinical studies have further shown that NF-κB RelA (p65) activation is associated with increased heparanase gene expression and correlated with poor clinical pathological characteristics in gastric cancers6, pancreatic adenocarcinoma7 and gallbladder carcinoma8. Consistent with this, our data demonstrated that JG3 decreased heparanase mRNA and protein expression by interfering with NF-κB activation. Additional genes, such as RhoA and COX2, which are involved in tumor metastasis and angiogenesis17, were also suppressed by JG3 treatment in a time-dependent manner. Our previously study showed that short-term treatment with JG3 inhibited heparanase activity by binding to the KKDC and QPLK domains within heparanase1. Taken together with our currently data, these results indicate that short-term treatment with JG3 can quickly inhibit heparanase activity by directly binding to it, whereas long-term treatment inhibits NF-κB activation and subsequently down-regulates expression of heparanase as well as other genes involved in metastasis and angiogenesis. The combined actions of these effectors serve to realize the tumor–growth inhibitory, anti-metastatic and anti-angiogenic functions of JG3.
Constitutive activation of NF-κB is prevalent in tumors and is reported to be associated with resistance to drug treatment and poor clinical prognosis18, 19, 20, 21. We found that JG3 preferentially inhibits the growth of tumors in which NF-κB is constitutively activated, highlighting the potential applicability of JG3 in cancer therapy. Our results further revealed that JG3 treatment apparently inhibited NF-κB activation by interfering with activation of upstream signaling components, although the precise mechanism is not yet clear. The actions of JG3 were also specific to the NF-κB pathway, and did not interfere with other transcription factors, such as STAT-3.
In sum, in this study we have further elucidated the mechanism underlying JG3 function and shown for the first time that JG3 has both in vitro and in vivo anti-NF-κB effects, preferentially inhibiting the growth of tumors in which NF-κB is constitutively activated. Considered in the context of the anti-angiogenic and anti-metastatic roles of JG3 involving heparanase, this additional anti-NF-κB mechanism highlights the importance of JG3 as a new lead molecule in cancer therapy.
Author contribution
Jing ZHANG, Mei-yu GENG, and Jian DING designed the study. Jing ZHANG performed experiments, analyzed the results and wrote the manuscript, which was revised by Mei-yu GENG and Jian DING. Xian-liang XIN synthesized compounds. Qiu-ning LI was involved in part of the study, and Ming LI contributed to immunohistochemistry assays. Yi CHEN and Li-ping LIN directed the in vivo study.
References
Zhao H, Liu H, Chen Y, Xin X, Li J, Hou Y, et al. Oligomannurarate sulfate, a novel heparanase inhibitor simultaneously targeting basic fibroblast growth factor, combats tumor angiogenesis and metastasis. Cancer Res 2006; 66: 8779–87.
Ilan N, Elkin M, Vlodavsky I . Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol 2006; 38: 2018–39.
Vlodavsky I, Goldshmidt O, Zcharia E, Atzmon R, Rangini-Guatta Z, Elkin M, et al. Mammalian heparanase: involvement in cancer metastasis, angiogenesis and normal development. Semin Cancer Biol 2002; 12: 121–9.
Goldshmidt O, Zcharia E, Abramovitch R, Metzger S, Aingorn H, Friedmann Y, et al. Cell surface expression and secretion of heparanase markedly promote tumor angiogenesis and metastasis. Proc Natl Acad Sci USA 2002; 99: 10031–6.
Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A . Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem 2005; 96: 897–905.
Andela VB, Schwarz EM, Puzas JE, O'Keefe RJ, Rosier RN . Tumor metastasis and the reciprocal regulation of prometastatic and antimetastatic factors by nuclear factor kappaB. Cancer Res 2000; 60: 6557–62.
Wu WJ, Pan CE, Liu QG, Meng KW, Yu HB, Wang YL, et al. Expression of heparanase and nuclear factor kappa B in pancreatic adenocarcinoma. Nan Fang Yi Ke Da Xue Xue Bao 2007; 27: 1267–70.
Wu W, Pan C, Yu H, Gong H, Wang Y . Heparanase expression in gallbladder carcinoma and its correlation to prognosis. J Gastroenterol Hepatol 2008; 23: 491–7.
Pacifico F, Leonardi A . NF-kappaB in solid tumors. Biochem Pharmacol 2006; 72: 1142–52.
Perkins ND . Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 2007; 8: 49–62.
Haffner MC, Berlato C, Doppler W . Exploiting our knowledge of NF-kappaB signaling for the treatment of mammary cancer. J Mammary Gland Biol Neoplasia 2006; 11: 63–73.
Bonizzi G, Karin M . The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 2004; 25: 280–8.
Ghosh S, Karin M . Missing pieces in the NF-kappaB puzzle. Cell 2002; 109 Suppl: S81–96.
Li QN, Liu HY, Xin XL, Pan QM, Wang L, Zhang J, et al. Marine-derived oligosaccharide sulfate (JG3) suppresses heparanase-driven cell adhesion events in heparanase over-expressing CHO-K1 cells. Acta Pharmacol Sin 2009; 30: 1033–8.
Khan N, Afaq F, Kweon MH, Kim K, Mukhtar H . Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res 2007; 67: 3475–82.
Sun C, Chan F, Briassouli P, Linardopoulos S . Aurora kinase inhibition downregulates NF-kappaB and sensitises tumour cells to chemotherapeutic agents. Biochem Biophys Res Commun 2007; 352: 220–5.
Karin M . Nuclear factor-kappaB in cancer development and progression. Nature 2006; 441: 431–6.
Van Waes C . Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res 2007; 13: 1076–82.
Zhou Y, Yau C, Gray JW, Chew K, Dairkee SH, Moore DH, et al. Enhanced NF kappa B and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer 2007; 7: 59.
Baldwin AS . Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest 2001; 107: 241–6.
Izzo JG, Correa AM, Wu TT, Malhotra U, Chao CK, Luthra R, et al. Pretherapy nuclear factor-kappaB status, chemoradiation resistance, and metastatic progression in esophageal carcinoma. Mol Cancer Ther 2006; 5: 2844–50.
Acknowledgements
The project was supported by Natural Science Foundation of China for Distinguished Young Scholars (No 30725046), National Basic Research Program Grant of China (No 2003CB716400), Natural Science Foundation of China for Innovation Research Group (No 30721005), the Knowledge Innovation Program of Chinese Academy of Sciences (No KSCX2-YWR-25), Key New Drug Creation and Manufacturing Program (No 2009ZX09103-073), 863 Hi-Tech Program of China (No 2006AA020602).
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary information
(A) structure of JG3. (B) NF-κB activation status in four tumor cell lines. (DOC 556 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Chen, Y., Xin, Xl. et al. Oligomannurarate sulfate blocks tumor growth by inhibiting NF-κB activation. Acta Pharmacol Sin 31, 375–381 (2010). https://doi.org/10.1038/aps.2010.13
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.13
Keywords
This article is cited by
-
Anticancer effects of marine compounds blocking the nuclear factor kappa B signaling pathway
Molecular Biology Reports (2022)
-
Angiosuppressive properties of marine-derived compounds—a mini review
Environmental Science and Pollution Research (2017)
-
Oligomannurarate sulfate inhibits CXCL12/SDF-1-mediated proliferation and invasion of human tumor cells in vitro
Acta Pharmacologica Sinica (2013)
-
Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide
BMC Medicine (2012)