Abstract
Aim:
To investigate the effects of docosahexaenoic acid (DHA) on large-conductance Ca2+-activated K+(BKCa) channels and voltage-dependent K+ (KV) channels in rat coronary artery smooth muscle cells (CASMCs).
Methods:
Rat CASMCs were isolated by an enzyme digestion method. BKCa and KV currents in individual CASMCs were recorded by the patch-clamp technique in a whole-cell configuration at room temperature. Effects of DHA on BKCa and KV channels were observed when it was applied at 10, 20, 30, 40, 50, 60, 70, and 80 μmol/L.
Results:
When DHA concentrations were greater than 10 μmol/L, BKCa currents increased in a dose-dependent manner. At a testing potential of +80 mV, 6.1%±0.3%, 76.5%±3.8%, 120.6%±5.5%, 248.0%±12.3%, 348.7%±17.3%, 374.2%±18.7%, 432.2%±21.6%, and 443.1%±22.1% of BKCa currents were increased at the above concentrations, respectively. The half-effective concentration (EC50) of DHA on BKCa currents was 37.53±1.65 μmol/L. When DHA concentrations were greater than 20 μmol/L, KV currents were gradually blocked by increasing concentrations of DHA. At a testing potential of +50 mV, 0.40%±0.02%, 1.37%±0.06%, 11.80%±0.59%, 26.50%±1.75%, 56.50%±2.89%, 73.30%±3.66%, 79.70%±3.94%, and 78.1%±3.91% of KV currents were blocked at the different concentrations listed above, respectively. The EC50 of DHA on KV currents was 44.20±0.63 μmol/L.
Conclusion:
DHA can activate BKCa channels and block KV channels in rat CASMCs, and the EC50 of DHA for BKCa channels is lower than that for KV channels; these findings indicate that the vasorelaxation effects of DHA on vascular smooth muscle cells are mainly due to its activation of BKCa channels.
Similar content being viewed by others
Introduction
ω-3 Polyunsaturated fatty acids (ω-3 PUFAs) consist mainly of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Recently, more attention has been paid to the beneficial effects of ω-3 PUFAs on risk reduction of cardiovascular diseases, especially with regard to vasodilatation, improvement of coronary artery blood flow, anti-arrhythmia, disease prevention, lowering of blood pressure, and other parameters1, 2. However, most of the studies mentioned above were performed in clinical practice, and the molecular mechanisms underlying the cardioprotective effects of ω-3 PUFAs are not fully understood. Studies have shown that dietary DHA in spontaneously hypertensive rats lowers blood pressure and reduces angiotensin II-mediated vasoconstriction. However, the ionic mechanisms driving these effects remain controversial3, 4.
It has been reported that there are at least four types of potassium channel in vascular smooth muscle cells (VSMCs): the calcium-activated potassium channel (KCa), the voltage-dependent potassium channel (KV), the ATP-sensitive potassium channel (KATP), and the inward rectifier potassium channel (Kir). These channels play important roles in cell membrane potential repolarization, resting potential maintenance, cell excitability, and the adjustment of VSMC contraction and relaxation. The channels also have important effects on vascular tone. KV channels are widely distributed in VSMCs, and they regulate the contraction and relaxation of VSMCs through effects on membrane potential and voltage-gated Ca2+ channel activity5. BKCa channels are most plentiful in VSMCs. Due to their large conductance and high density in VSMCs, BKCa channels are very important in controlling resting membrane potential and vascular tone6, 7, 8. It has been reported that the arachidonic acid (AA) pathway and the DHA metabolizer of cytochrome P450 (CYP450) pathway are potent vasodilators that directly activate BKCa channels in the rat CASMC9. Most importantly, the BKCa channel was more sensitive to n-3 eposide than to n-6 eposide. Hence, the DHA metabolites of CYP450 are known to be the most potent vascular BKCa channel activators and vasodilators thus far recognized. It has also been shown that the effects of DHA on Kv channels are controversial in different kinds of cell, including ventricular myocytes, neurons, and vascular smooth muscle cells (VSMCs)10, 11. In this study, we investigated whether DHA had a direct effect on BKCa channels or other vascular K+ channels. We examined the effects of DHA on BKCa channels and voltage-dependent K+ (KV) channels from freshly isolated CASMCs of rats, using whole-cell patch-clamp recordings to study the mechanism for DHA-induced vasodilatation.
Materials and methods
Major experimental instruments
The instruments included MultiClamp 700B patch clamp amplifier (Axon Instruments, USA), D/A and A/D converter (DigiData 1322, Axon Instruments, USA), Pclamp9.0 pulse software (Axon Instruments, USA), MP-285 motorized micromanipulator (Sutter Instruments, USA), IX71 inverted microscope (Olympus, Japan), SA-OLY/2 and DH-35 culture dish heater (Warner Instruments, USA), and P-97 micropipette puller (Sutter Instruments, USA).
Reagents, solutions and drugs
Trypsin inhibitor (type II-S) was from Sigma-Aldrich; papain and dithiothreitol were from Biosharp (Korea); collagenase was from Worthington Biochemicals. The buffer solution contained (in mmol/L) 145.0 NaCl, 4.0 KCl, 0.05 CaCl2, 1.0 MgCl2, 10.0 HEPES, and 10.0 glucose, adjusted to pH 7.2 with NaOH. For the recording of BKCa currents, the pipette solution contained (in mmol/L) 140 KCl, 0.5 MgCl2, 5.0 Na2ATP, 0.5 Na2GTP, 10 HEPES, and 1.0 EGTA, at a pH of 7.2. CaCl2 was added to provide 1.0 μmol/L of free Ca2+, and the external solution was the buffer solution. For the recording of KV current, the pipette solution contained (in mmol/L) 110 KCl, 30 KOH, 10 HEPES, 10 EGTA, 1 MgCl2, 1 CaCl2, 3 Na2ATP, and 0.5 GTP, adjusted to pH 7.2 with KOH. To minimize the activity of KATP channels, a high concentration of ATP (3.0 mmol/L) was used. The free [Ca2+] was 20 nmol/L, calculated using the Maxchelator program; the external solution contained (in mmol/L) 134 NaCl, 6 KCl, 1 MgCl2, 0.1 CaCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.4 with NaOH. To minimize the activity of BKCa channels, external Ca2+ levels were lowered to 100 nmol/L to reduce Ca2+ influx. Intracellular Ca2+ was buffered to a low level (20 nmol/L) with EGTA. DHA was dissolved in absolute ethanol as a 50 mmol/L stock solution, protected from light, and stored at −20°C. On the day of the experiment, portions of these stock solutions were added to the bath solution to obtain the desired final concentrations.
CVSMCs isolation
Healthy Sprague-Dawley rats of both sexes, aged 8–12 weeks and weighing approximately 200 g, were provided by the Experimental Animal Center of Soochow University (Suzhou, China). All of the investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the PRC National Department of Health. Animals were anesthetized with pentobarbital sodium intraperitoneally (50 mg/kg, ip), and the heart was rapidly removed from the thorax and placed in a buffer solution. CVSMCs in small arteries were isolated as described previously12, 13. In brief, after being isolated under a dissection microscope, the secondary and tertiary branches of the coronary arteries were incubated at 37 °C for 10 min in 1.0 mL of buffer solution containing 0.1% bovine serum albumin. Initially, vessels were enzymatically digested for 10 min at 37 °C in 1.0 mL of fresh buffer solution containing 1.5 mg of papain and 1.0 mg of dithiothreitol. The vessels were further digested for 10 min at 37 °C in 1.0 mL of fresh buffer solution containing 1.0 mg of collagenase and 1.0 mg of a trypsin inhibitor. To remove the exogenous enzymes, each vessel was transferred three times to 1.0 mL of fresh buffer solution and gently triturated with a fire-polished glass pipette until complete dissociation. The resulting smooth muscle cell suspension was stored at 4 °C and used within 8 h.
BKCa and KV current recording and channel kinetic parameters
BKCa and KV currents in individual CVSMCs were recorded using a patch-clamp technique following the method described previously14, 15, 16. In brief, isolated CVSMCs were placed in a 1.0 mL chamber on the stage of an inverted microscope and perfused with the external solution. Borosilicate glass capillaries were used to fabricate patch pipettes. When filled with internal solution, each electrode had a tip resistance between 4 and 10 MΩ and a typical seal resistance greater than 10 GΩ. BKCa currents were recorded using a MultiClamp 700B patch clamp amplifier. Voltage clamp pulses were generated via an IBM-compatible computer connected to a DigiData 1322 D/A and A/D converter. Data acquisition and analyses were performed using pCLAMP9.0 software. The effects of DHA at different concentrations (10, 20, 30, 40, 50, 60, 70, and 80 μmol/L) on BKCa currents were measured by eliciting BKCa currents in the presence of 1 μmol/L cytoplasmic free Ca2+. Responses were measured from a holding potential of −60 mV to a testing potential of +80 mV at 10 mV increments for 400 ms, and they were repeated at 10-s intervals. To eliminate KV current contamination, the external solution included 3 mmol/L 4-AP, a Kv channel blocker. KV currents were generated by stepwise 10 mV depolarizing pulses (400 ms duration, 10-s intervals) from a holding potential of −60 mV to +50 mV in cells dialyzed with 100 nmol/L ionized Ca2+. The effects of DHA on KV channel currents at the concentrations listed above were measured. In addition, 100 nmol/L of iberiotoxin (IBTX), a BKCa channel blocker, was present in the external solution to block BKCa channel activities. Currents were normalized to cell capacitance and expressed as pA/pF. All experiments were performed at room temperature (21–23 °C).
Statistical analysis
Continuous variables are expressed as mean±standard deviation. SPSS11.5 (Chicago, Illinois, USA) was used for the statistical analysis. Comparisons among groups were performed by an analysis of variance (ANOVA) with a least-significant difference contrast. Control and drug data for individual groups were compared using paired t-tests. P<0.05 was considered significant. OriginPro7.5 software (OriginLab, USA) was utilized to calculate the EC50.
Results
Effects of DHA on BKCa currents
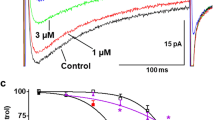
(1) Effects on BKCa currents and peak currents: at a testing potential of +80 mV, the stable peak current was 513.5±101.96 pA and current density was 48.9±2.45 pA/pF (n=10). When DHA was applied at 10, 20, 30, 40, 50, 60, 70, and 80 μmol/L, BKCa current increases of 6.1%±0.3%, 76.5%±3.8%, 120.6%±5.5%, 248.0%±12.3%, 348.7%±17.3%, 374.2%±18.7%, 432.2%± 21.6%, and 443.1%±22.1%, respectively, were observed (P<0.05, n=10). Figure 1 shows current traces elicited by depolarization from the −60 mV holding potential (HP) to the +80 mV test potential at different DHA concentrations. The EC50 of the BKCa current, calculated by a curve fit with the concentrations and percentage increases for the currents, was 37.53±1.65 μmol/L (Figure 2). (2) Effects on the current-voltage (I–V) curves of BKCa currents: When DHA was used at 20, 40, 60, and 80 μmol/L, BKCa currents and densities increased gradually with increasing DHA concentrations. The I–V curves were shifted upward (Figure 3).
Effects of DHA on KV currents
(1) Effects on KV currents and peak currents: At a testing potential +50 mV, the stable peak current was 460.95±73.01 pA and current density was 43.9±2.32 pA/pF (n=10). When DHA was applied at 10, 20, 30, 40, 50, 60, 70, and 80 μmol/L, KV currents were blocked by 0.40%±0.02%, 1.37%±0.06%, 11.80%±0.59%, 26.50%±1.75%, 56.50%±2.89%, 73.30%±3.66%, 79.70%± 3.94%, and 78.10%±3.91%, respectively (P<0.05, n=10). Figure 4 shows the current traces elicited by depolarization from the -60 mV holding potential to the +50 mV test potential at different DHA concentrations. The EC50 of DHA on KV currents was 44.20±0.63 μmol/L (Figure 5). (2) Effects on the I–V curves of KV currents: when DHA was used at concentrations of 20, 40, 60, and 80 μmol/L, KV currents and current densities decreased gradually with increasing DHA concentrations. The I–V curves were shifted downward (Figure 6).
Discussion
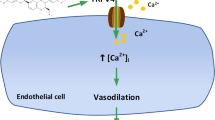
Many kinds of potassium channels in VSMCs play the important role in regulating membrane potential and vascular tone. Of these potassium channels, BKCa channels and KV channels are widely distributed in VSMCs. In particular, BKCa channels are most widely expressed channels in VSMCs. BKCa channels are composed of a pore-forming α-subunit and regulatory β-subunit. Because of their strong conductance (200–250 pS) and high density (1–4 channels/μm2) in VSMCs, the activity of BKCa channels is a key determinant in controlling resting membrane potential and vascular tone17, 18. BKCa channels can be activated by either elevation of cytosolic Ca2+ concentrations ([Ca2+]i) or cell membrane depolarization19, 20. The activation of BKCa channels repolarizes the cell membrane potential and promotes the closure of voltage-activated Ca2+ channels (VACC), opposing the role of VACCs in vasoconstriction. Therefore, BKCa channels are key channels for the regulation of vascular tone in VSMCs. Interestingly, BKCa channels are not expressed in cardiomyocytes21. KV channels are also composed of a pore-forming α-subunit and regulatory β-subunit. To date, more than 30 genes encoding several subfamilies of KV α-subunits are currently recognized, and each α-subunit is associated with ancillary β-subunits that influence the characteristics of the channel and modify the intrinsic properties of the α-subunits22, 23. Studies have shown that the Kv1.2, Kv1.3, Kv1.4, Kv1.5, Kv4.1, and Kv4.2 genes are expressed in rat VSMCs as well as rat ventricular myocytes. With regard to the rat coronary artery, the expression of Kv1.2 and Kv1.5 has been found in rat CASMCs24, 25, 26.
Broad KV channel distribution has also been detected in VSMCs. Depolarization of the membrane potential in VSMCs leads to an influx of Ca2+ through L-type Ca2+ channels and activation of the contractile machinery. These changes permit KV channels to open and allow an efflux of K+, resulting in repolarization and the consequent return to the resting membrane potential. Taken together, these findings indicate that the main function of KV channels is to limit membrane depolarization and maintain resting vascular tone22, 27.
Fatty acids, especially ω-3 PUFAs, are one important source of cardiac energy. They have important effects on the electrophysiology and metabolism of cardiomyocytes. It has been reported that a high dietary intake of ω-3 PUFA (more than 4.0 g a day) is linked to favorable physiological alterations, including reduction in triglyceride levels, anti-arrhythmic actions, anti-thrombotic effects, lowering of blood pressure, and enhancement immune function; together, these changes may contribute to a lower risk of cardiovascular diseases2, 28, 29. Although some clinical studies have elucidated that DHA intake accumulates easily in cardiac tissues, leads to the dilation of the coronary artery, and enhances coronary artery blood flow30, the mechanisms of these beneficial effects of DHA for the prevention of cardiovascular disease are still not completely clear. To elucidate the mechanisms by which DHA affects vasodilatation, we investigated the effects of DHA on BKCa and KV channels in rat CASMCs using the whole-cell patch clamp technique.
Our results have shown that DHA at concentrations greater than 10 μmol/L activated BKCa channels, and BKCa currents increased with the elevation of DHA concentrations. This indicates that DHA has an activating effect on BKCa channels, which then leads to the efflux of K+, closure of Ca2+ channels, reduction of influx of Ca2+, acceleration of cell membrane potential repolarization, and vasodilatation. More interestingly, our results have shown that DHA has a completely opposite effect on KV channels. When DHA concentrations were greater than 20 μmol/L, KV currents were gradually blocked by increasing concentrations of DHA; this finding indicates that DHA can inhibit KV channels. Theoretically, the inhibition of KV channels can block the efflux of K+ and repolarization of the cell membrane potential, which results in vasoconstriction. However, many studies have shown that DHA has the characteristics to induce vasodilatation6, 8, 12, 30, 31. Because 1) the density of BKCa channels is far greater than that of KV channels in VSMCs and 2) the EC50 of DHA on BKCa channels is less than that on KV channels, we suggest that the increased amount of K+ efflux through activated BKCa channels is far greater than the reduced amount of K+ efflux through inhibited KV channels at a given DHA concentration. Consequently, we conclude that the effect of DHA on vasodilatation is accomplished mainly via the activation of BKCa channels, and that BKCa channels thus play a key role in vasodilatation as well as in improvement of coronary artery blood flow.
Ye et al.12 tested the potency of docosahexaenoate and its five cytochrome P-450 epoxygenase metabolites, known as the epoxydocosapentaenoates (EDPs), in dilating porcine and rat coronary arterioles. They found that, compared with 13,14-EDP, docosahexaenoate (DHA) weakly activated BKCa channels in a single channel recording. However, these authors used low concentrations of DHA (below 10 μmol/L). Another study31 has shown that DHA can significantly dilate aortic rings freshly isolated from spontaneously hypertensive rats through the modulation of L-type Ca2+ channels and KATP channels. Due to the absence of a metabolic environment under these conditions, metabolites of DHA are absent; therefore, DHA may have direct effects on K+ channels or L-type Ca2+ channels in rat VSMCs. The results from our study have shown that DHA at concentrations greater than 10 μmol/L directly and markedly activated BKCa channels. With regard to the effects of DHA on KV channels, varying results have been reported. Wu et al.11 demonstrated that the enhancement of outward voltage-gated K+ (IKV) currents and inwardly rectifying IK1 currents could be related to vasorelaxation induced by DHA in human coronary artery smooth muscle, and Poling et al.10 reported that DHA inhibited KV channels in neurons. Studies have also shown that DHA inhibits KV channels in ventricular myocytes, in which the expression of KV1.1-KV1.5 and KV4.1-KV4.2 has been detected32, 33. Our studies have shown that DHA at concentrations greater than 20 μmol/L markedly inhibited KV channels in rat CASMCs.
Studies have shown that several types of vascular K+ channels, including BKCa channels and KV channels, are activated by activating adenylyl cyclase. This activation thereby increases the intracellular concentration of cAMP, which activates a cAMP-dependent protein kinase (PKA). The activation of PKG can also result in the activation of vascular K+ channels34. In addition, inhibition of K+ channels in VSMCs is initiated through the activation of protein kinase C (PKC)35, 36. However, the molecular mechanisms of BKCa channel activation and KV channel inhibition by DHA remain unclear.
In our experiments, DHA directly activated BKCa channels, indicating that DHA may bind on or be close to the α-pores or β-regulatory units of BKCa channels and induce conformational changes in BKCa channels. The molecular mechanisms by which BKCa channels are activated and KV channels inhibited by DHA require future study.
Because there are still no reports regarding the direct effects of DHA on BKCa channels and KV channels in rat CASMCs, the present study has an important implication for the investigation of the mechanisms by which ω-3 PUFA causes vasodilatation and the lowering of blood pressure. However, our studies have some limitations. First, only the effects of DHA on BKCa and KV channels were investigated, whereas the effects of DHA on KATP and Kir channels need to be probed further. Second, because ω-3PUFAs consist mainly of DHA and EPA, the effects of EPA on BKCa and KV channels need to be studied further.
Author contribution
Wen-ping JIANG designed research; Li-hong LAI performed research; Ru-xing WANG contributed new analytical tools and reagents; Li-hong LAI analyzed data and wrote the paper.
References
Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ . Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation 2000; 102: 1264–9.
Clemens von Schacky C, Harris WS . Cardiovascular benefits of omega-3 fatty acids. Cardiovasc Res 2007; 73: 310–5.
Engler MM, Engler MB, Pierson DM, Molteni LB, Molteni A . Effects of docosahexaenoic acid on vascular pathology and reactivity in hypertension. Exp Biol Med (Maywood) 2003; 228: 299–307.
Frenoux JM, Prost ED, Belleville JL, Prost JL . A polyunsaturated fatty acid diet lowers blood pressure and improves antioxidant status in spontaneously hypertensive rats. J Nutr 2001; 131: 39–45.
Robert HC . Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys 2005; 42: 167–95.
Wu SN . Large-conductance Ca2+-activated K+ channels: physiological role and pharmacology. Curr Med Chem 2003; 10: 649–61.
Calderone V . Large-conductance Ca2+-activated K+ channels: function, pharmacology and drugs. Curt Med Chem 2002; 9: l385–95.
Ko EA, Han J, Jung ID, Park WS . Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res 2008; 44: 65–81.
Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M . EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circ Physiol 2001; 280: 2430–40.
Poling JS, Vicini S, Rogawski MA, Salem N Jr . DHA block of neuronal voltage-gated K+ channels: subunit selective antagonism by zinc. Neuropharmacology 1996; 35: 969–82.
Wu KT, Huang CT, Wei J, Tsait LM, Hsu CH, Chen YC, et al. Vasodilator action of docosahexaenoic acid (DHA) in human coronary arteries in vitro. Chin J Physiol 2007; 50: 164–70.
Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M . Cytochrome P-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther 2002; 303: 768–76.
Lu T, Wang XL, He T, Zhou W, Kaduce TL, Katusic ZS, et al. Impaired arachidonic acid-mediated activation of large-conductance Ca2+-activated K+ channels in coronary arterial smooth muscle cells in Zucker diabetic fatty rats. Diabetes 2005; 54: 2155–63.
Wang XL, Ye D, Timothy E, Cao S, Shah VH, Katusic ZS, et al. Caveolae targeting and regulation of large conductance Ca2+-activated K+ channels in vascular endothelial cells. Biol Chem 2005; 3: 11656–64.
Bubolz AH, Li HW, Wu QP, Liu YP . Enhanced oxidative stress impairs cAMP-mediated dilation by reducing KV channel function in small coronary arteries of diabetic rats. Am J Physiol Heart Circ Physiol 2005; 289: H1873–H1880.
Hayabuchi Y, Standen NB, Davies NW . Angiotensin II inhibits and alters kinetics of voltage gated K+ channels of rat arterial smooth muscle. Am J Physiol Heart Circ Physiol 2001; 281: H2480–H2489.
Cox RH, Rusch NJ . New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation 2000; 29: 243–57.
Tanaka Y, Koike K, Toro L . MaxiK channel roles in blood vessel relaxations induced by endothelium-derived relaxing factors and their molecular mechanisms. J Smooth Muscle Res 2004; 40:125–53.
Latorre R, Brauchi S . Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res 2006; 39: 385–401.
Park WS, Son YK, Kim NR, Youm JB, Warda M, Ko JH, et al. Direct modulation of Ca2+-activated K+ current by H-89 in rabbit coronary arterial smooth muscle cells. Vascul Pharmacol 2007; 46: 105–13.
Birgit E, Dobromir D . Vascular large conductance calcium-activated potassium channels: Functional role and therapeutic potential. Naunyn-Schmiedeberg's Arch Pharmacol 2007; 376: 145–55.
Korovkina VP, England SK . Molecular diversity of vascular potassium channel isoforms. Clin Exp Pharmacol Physiol 2002; 29: 317–23.
Bahring R, Milligan CJ, Vardanyan V, Engeland B, Young BA, Dannenberg J, et al. Coupling of voltage-dependent potassium channel inactivation and oxidoreductase active site of KV beta subunits. J Biol Chem 2001; 276: 22923–9.
Robert HC . Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys Volume 2005; 42: 167–95.
Gautier M, Hyvelin JM, de Crescenzo V, Eder V, Bonnet P . Heterogeneous KV1 function and expression in coronary myocytes from right and left ventricles in rats. Am J Physiol Heart Circ Physiol 2007; 292: H475–H482.
Hyvelin JM, Gautier M, Lemaire MC, Bonnet P, Eder V . Adaptative modifications of right coronary myocytes voltage-gated K+ currents in rat with hypoxic pulmonary hypertension. Pflugers Arch 2008; 17: 424–34.
Sobey CG . Potassium channel function in vascular disease. Arterioscler Thromb Vasc Biol 2001; 21: 28–38.
Calder PC . n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 2004; 107: 1–11.
Frenoux JM, Prost ED, Belleville JL, Prost JL . A polyunsaturated fatty acid diet lowers blood pressure and improves antioxidant status in spontaneously hypertensive rats. J Nutr 2001; 131: 39–45.
Watkins SM, Lin TY, Davis RM, Ching JR, DePeters EJ, Halpern GM, et al. Unique phospholipid metabolism in mouse heart in response to dietary docosahexaenoic or α-linolenic acids. Lipids 2001; 36: 247–54.
Mary B, Marguerite M . Docosahexaenoic acid-induced vasorelaxation in hypertensive rats: mechanisms of action. Biol Res Nurs 2000; 2: 85–95.
Xiao YF, Morgan JP, Leaf A . Effects of polyunsaturated fatty acids on cardiac voltage-activated K+ currents in adult ferret cardiomyocytes. Sheng Li Xue Bao 2002; 54: 271–81.
Guizy M, David M, Arias C, Zhang L, Cofán M, Ruiz-Gutiérrez V, et al. Modulation of the atrial specific KV1.5 channel by the n-3 polyunsaturated fatty acid, alpha-linolenic acid. J Mol Cell Cardiol 2008; 44: 323–35.
Ledoux J, Werner ME, Brayden JE, Nelson MT . Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006; 21: 69–78.
Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F . Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase C. Circ Res 2003; 93: 656–63.
Crozatier B . Central role of PKCs in vascular smooth muscle cell ion channel regulation. J Mol Cell Cardiol 2006; 41: 952–5.
Acknowledgements
This work was supported in part by a grant (No CS20010015) from the Wuxi Science and Technology Bureau of Jiangsu Province, China and a grant from Project 135 of Key Laboratory, Jiangsu Province, China.
The authors thank Miss Hong-xia LI and Miss Lian-hua HAN for their assistance in the preparation of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, Lh., Wang, Rx., Jiang, Wp. et al. Effects of docosahexaenoic acid on large-conductance Ca2+-activated K+ channels and voltage-dependent K+ channels in rat coronary artery smooth muscle cells. Acta Pharmacol Sin 30, 314–320 (2009). https://doi.org/10.1038/aps.2009.7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2009.7