Abstract
Aim:
Arbidol is an immunomodulator that was first developed in Russia. In this study, we report the antiviral activity of arbidol against Hantaan virus (HTNV) in vitro and in vivo.
Methods:
The antiviral activity of arbidol in vitro was determined by plaque-forming assay, ranging from 0.5 to 8 μg/mL. To investigate whether arbidol has an antiviral effect in vivo, suckling BALB/c mice infected with HTNV were treated with arbidol at 24 h before infection with a 5, 10 or 20 mg·kg−1·d−1, once per day, for 10 days. On day 12 and 28 post infection (pi), histopathological changes and viral antigen were detected. On days 4, 8, 12, and 16 pi, the viral load of target organs and serum TNF-α levels of arbidol-treated animals were determined.
Results:
Arbidol was found to have potent inhibitory activity against HTNV when added in vitro before or after viral infection, with a 50% inhibitory concentration (IC50) of 0.9 and 1.2 μg/mL, respectively. The 50% lethal dose (LD50) of arbidol for suckling mice was 78.42 mg·kg−1·d−1. Oral administration of arbidol increased both survival rate and mean time to death (MTD). Treatment with arbidol reduced histopathological changes, decreased viral load and viral antigen levels, and modulated the level of serum TNF-α.
Conclusion:
Arbidol has the ability to elicit protective antiviral activity against HTNV in vivo and in vitro.
Similar content being viewed by others
Introduction
Hantaviruses are enveloped viruses of the genus Hantavirus, family Bunyaviridae, with a genome consisting of three segments of negative-sense RNA. The large (L), small (S), and medium (M) segments encode RNA-dependent RNA polymerase, the nucleocapsid (N) protein, and two envelope glycoproteins (G1 and G2), respectively1. Hantaviruses are transmitted to humans via inhalation of aerosolized excreta from infected rodents. The infection can lead to hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS)1, 2. More than 10 000 cases of HFRS are reported annually worldwide, mostly in Asia and Europe3, 4. Lethality ranges from 1% for Puumala virus (PUUV) and Seoul virus infections to approximately 15% for Hantaan (HTNV) virus infections5, 6, 7, 8.
Vaccination is the preferred method for controlling HFRS and reducing the impact of incidence. Several inactivated HTNV and Seoul virus vaccines for HFRS have been used8, but they are not yet completely effective or protective against all strains. Additionally, not all people at risk can receive the vaccination, especially those in regions having only sporadic outbreaks.
During the past few years, extensive efforts have been made in the search for effective anti-HTNV agents. Some nucleoside analogs have been found to be highly inhibitory in animal models9, but ribavirin is the only antiviral agent known to be successful against HTNV infections in clinical trails10. However, the utilization of ribavirin is limited by its myelosuppression and toxicity11, 12, 13. No antiviral agent has been approved by the Food and Drug Administration (FDA) to treat HFRS5. Therefore, an effort to seek alternative agents is strongly desirable.
Arbidol (ARB; 1H-indole-3-carboxylic acid, 6-bromo-4-[(dimethylamino)-methyl]-5-hydroxy-1-methyl-2-[(phenylthio) methyl]-, ethylester, monohydrochloride) is an immunomodulator that was developed in Russia14. It has been shown to have broad antiviral effects against viruses such as influenza virus, coxsackievirus, respiratory syncytial virus15, and hepatitis C virus (HCV)16. The antiviral mechanisms of arbidol may be due to increased interferon production or inhibition of enveloped virus fusion within endosomes17. The aim of the present study was to evaluate the anti-HTNV activity of arbidol in vivo and in vitro.
Materials and methods
Virus
The 76–118 strain of Hantaan virus was obtained from the Institute of Virology, Chinese Academy of Preventive Medicine, Beijing, China. The virus was propagated five times in Vero E6 cells (ATCC CRL 1586) to achieve a titer of 1×106 TCID50/0.1 mL. For animal inoculation, viral stock was diluted in minimal essential medium (MEM) (containing 2% FCS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin) and stored in multiple single-use aliquots at −80 °C.
Reagents and compounds
Arbidol (obtained from Qianjiang Pharmaceutical Co, LTD, Hubei, China) was initially dissolved in dimethyl sulfoxide (DMSO) and then further diluted with MEM containing 2% FCS (2% MEM) for testing in vitro. The final maximum DMSO concentration was 0.05%, and an equivalent amount of DMSO was added to all no-drug control samples. Arbidol was suspended in 0.5% sterilized methylcellulose for testing in vivo. A monoclonal antibody that targets nucleocapsid protein (A35, provided by the Institute of Virology, Chinese Academy of Preventive Medicine, Beijing, China) was used as the primary antibody for indirect immunofluorescence at a 5 μg/mL concentration. The second antibody was a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (R&D Systems, Minneapolis, MN).
In vitro evaluation of arbidol
Cell viability assay
To determine the cytotoxic concentration of arbidol, monolayers of confluent Vero E6 cells were exposed to various concentrations of arbidol in 2% MEM, and the dilution medium with 0.05% DMSO was used as the control. After 10 days of incubation, cell viability was examined by the MTT colorimetric assay18. The median cytotoxic concentration (CC50) was the concentration (μg/mL) required to reduce cell viability by 50%.
Viral yield reduction assay
Confluent Vero E6 cells were infected with 100 TCID50/0.1 mL of HTNV. After 2 h of adsorption at 35 °C, the cells were covered with 2% MEM containing varying concentrations of arbidol. At 10 days pi, cultures were subjected to two cycles of freezing and thawing, followed by centrifugation at low speed (1000×g), and the supernatants were titered by plaque assay19, 20. In brief, supernatants of serial 10-fold dilutions were inoculated onto Vero E6 cell monolayers in 24-well plates and then overlaid with agarose overlay medium. After incubation for 5 days at 35 °C, a second layer of agarose including neutral red (0.167 mg/mL) was added to the well and incubated for an additional 2 days at 35 °C in 5% CO2 atmosphere. After incubation, the number of plaques was counted. Antiviral activity was defined as 50% inhibitory concentration (IC50), ie, the concentration required to reduce viral yield by 50% compared with the untreated control cultures. Thus, the selective index (SI) for arbidol was determined from CC50/IC50.
Pre-treatment assay
To evaluate the effects of arbidol on prophylaxis for cell infection, serial two-fold dilutions of arbidol were dissolved in 2% MEM and incubated with cells for 24 h at 35 °C in 5% CO2 atmosphere. After removal of the compound, the cells were washed twice with phosphate buffered saline (PBS) and the cell monolayers were infected with 100 TCID50/0.1 mL of HTNV. The cell monolayers were grown in 2% MEM (including 0.05% DMSO) and samples grown in 0.05% DMSO without arbidol were used as the control. Ten days after inoculation, cultures were frozen and viral yields were determined by plaque assay. The IC50s were determined as described above.
Direct virucidal assay
Viral suspensions containing 100 TCID50/0.1 mL of viral stock were incubated with an equal volume of medium with or without serial two-fold dilutions of arbidol for 2 h at 35 °C. Duplicate cultures of confluent Vero E6 cells were infected with 200 μL mixed suspension. The inoculate was removed at 2 h post-infection and maintained with medium containing 2% FCS at 35 °C for 10 days. The viral yield of the collected fractions was determined by plaque assay.
Antiviral activity in vivo
Animals
Specific-pathogen-free pregnant BALB/c mice were obtained from the Animal Research Center of Wuhan University. Animals were assigned to individual cages and given food and water. They were observed daily, and births were timed to the nearest day. Based on the 50% lethal dose (LD50) of HTNV intracranial (ic) inoculation in suckling mice, 1- to 2-day-old mice were intracranially inoculated with 100 LD50 of viral stock to determine an acceptable infection dose. All work with these animals was performed in the Animal Biosafety Level 3 (ABSL-3) Laboratory of the Animal Research Center at Wuhan University and was approved by the Institutional Animal Care and Use Committee.
In vivo toxicity determinations
Arbidol was evaluated for the dose considered lethally toxic in 1-day-old mice. The doses studied were 160, 80, 40, 20 and 10 mg·kg−1·d−1. Normal controls received 0.5% methylcellulose solution instead of arbidol. Mice (n≥9) were treated by oral gavage once a day for 10 days. Animal weights were determined every day. They were observed for death daily for a total of 20 days.
Experimental design for animal studies
Suckling mice (2 days old) were inoculated ic with 100 LD50 of viral stock. The mice were randomly divided into five groups (35 mice per group) and arbidol was tested with the following dosage regimens: 5, 10, or 20 mg·kg−1·d−1 once a day for 10 days. Treatment was initiated at 24 h before infection, by the same investigator, and with the dose corrected for weight change. Placebo controls (inoculated with 100 LD50 of HTNV) and normal controls (inoculated with 2% MEM) received 0.5% methylcellulose solution instead of arbidol. Animals were observed twice daily and weighed once daily. Mice were observed daily for mortality for 28 days after infection. Death occurring 24 h after viral inoculation was considered traumatic, and such animals were considered withdrawals. The effect of arbidol was estimated using the reduction in the rate of mortality and prolongation of mean time to death (MTD). Four mice were sacrificed for each experimental point (days 4, 8, 12, and 16 pi), and survivors were euthanized on day 28 pi. Sera were pooled for all studies. Brains, lungs, and kidneys were aseptically dissected from the animals and divided into three parts: one-third was frozen for antigen detection by indirect immunofluorescence (IFA), one-third was placed in formalin for histological examination, and the rest was stored at -80 °C for virological parameter determination.
Histological study
Four animals were sacrificed on day 12 pi, and the survivors were euthanized on day 28 pi. Brain, lung, and kidney slices were fixed in 10% formalin, dehydrated in ethanol, and embedded in paraffin. Sections of 5 μm thickness were stained with hematoxylin and eosin (H&E).
IFA
On day 12 pi, four animals were sacrificed, and the survivors were euthanized on day 28 pi. Frozen sections of brain, lung, and kidney were prepared in a cryostat. IFA was carried out as reported previously20 with the N-specific monoclonal antibody A35. Specific reactions between A35 and N protein were detected with FITC-conjugated goat anti-mouse IgG and observed with fluorescent microscopy (Olympus CX-41, Japan).
RT-PCR
On days 4, 8, 12, and 16 pi, total RNA was isolated from the lungs, brains, and kidneys of HTNV-infected mice using TRIzol® Reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. RNA (1.0 μg) was reverse transcribed in a reaction volume of 20 μL. Synthesis of cDNA was carried out at 42 °C for 60 min, followed by a denaturation step at 95 °C for 5 min. The reverse transcription products served as templates for PCR. The PCR protocol consisted of 30 cycles of denaturation (94 °C for 1 min), annealing (55 °C for 2 min), and extension (72 °C for 3 min), using a Thermal Cycler (Eppendorf, German). The S segment of the viral genome was chosen for detection and the primer sequences used were 5′-TGA GAA ATG TGT ATG ACA tGA-3′ (sense) and 5′-ACT AGA CAC TGT TTC AAA TGA-3′ (antisense). The parallel expression of β-actin mRNA was tested under the same PCR conditions as an internal standard, using the primers 5′-TCA TCA CTA TTG GCA ACG AGC-3′ (sense) and 5′-AAC AGT CCG CCT AGA AGC AC-3′ (antisense). For quantification, standard curves were generated to show that PCR detection was in the linear range. PCR bands on the gel were scanned using a computer analysis system (Bandscan 5.0, Glyko), and the viral mRNA signal was normalized relative to the corresponding β-actin mRNA signal from the same sample. Data are presented as the mRNA/β-actin ratio.
Lung, kidney and brain viral titers determination
At days 4, 8, 12, and 16 pi, the organs of four mice from each group were homogenized to 10% (weight/volume) suspensions in test medium. The homogenates were subjected to two cycles of freezing and thawing followed by centrifugation at low speed (1000×g). The titers of infectious viral particles were determined by the standard plaque formation assay.
Detection of TNF-α in serum by enzyme-linked immunosorbent assay (ELISA)
Blood was obtained at days 4, 8, 12, and 16 pi and the serum stored at −80 °C. TNF-α was detected in the serum by QuantiKine quantitative sandwich enzyme immunoassay (EIA) (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Statistical analysis
The data were analyzed using SPSS 16.0 software. One-way ANOVA was used to determine statistical differences for MTD, serum TNF-α level, and mRNA/β-actin ratios. A log rank test was used to evaluate the survival distributions for the different levels of group. Any P value less than 0.05 was considered to indicate statistical significance.
Results
Inhibition of HTNV in vitro
Vero E6 cells were treated with arbidol concentrations in the range of 1 to 20 μg/mL and cell viability was investigated by MTT assay. Arbidol did not significantly affect the viability of cells at concentrations lower than 8 μg/mL and the CC50 of arbidol was 15.64 μg/mL. We therefore examined the anti-HTNV activity of arbidol at concentrations of 8 μg/mL or lower. The results are summarized in Table 1. Overall, arbidol was efficacious against HTNV when added before or after viral infection, with mean IC50 values determined by use of plaque-forming assay. No directly virucidal effects of arbidol were found at concentrations lower than 8 μg/mL in this experiment.
Efficacy of arbidol on lethal HTNV infections in suckling mice
The LD50 of arbidol
Oral gavage treatment with arbidol for 10 days indicated that the LD50 of arbidol was 78.42 mg·kg−1·d−1. It should be noted that no obvious weight loss was seen at dosages below 40 mg·kg−1·d−1. No attempt was made to determine the cause of death for the mice in this range-finding study.
Protective efficacy of arbidol in suckling mice
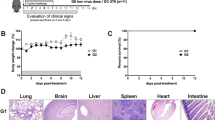
Suckling mice were infected with HTNV as described above and treated with the following dosage regimens: 5, 10, or 20 mg·kg−1·d−1 once a day for 10 days. Animals were observed and weighed individually every day. The clinical symptoms evaluated in these animals included transient state of hyperactivity, weight loss, and ruffled appearance of their coats accompanied by hunched posture and progressively diminishing mobility. Paralysis of both hind limbs appeared prior to death. Compared with the placebo-treated animals, arbidol-treated animals died later and appearance of clinical signs was reduced in a dose-dependent manner. Weight recovered and symptoms disappeared by day 28 in the survivors (Figure 1). All placebo-treated mice died, with an MTD of 17.17 days whereas treatment with arbidol increased both survival rate and MTD (Figure 1). Arbidol at 10 mg·kg−1·d−1 increased the survival rate to 33.3% and MTD to 21.5 days (P<0.05) and at 20 mg·kg−1·d−1 increased the survival rate to 60% and MTD to 23.8 days (P<0.05).
Effects of orally administered arbidol on the lethal HTNV challenge model. Arbidol at 5, 10, and 20 mg·kg−1·d−1 was orally administered 24 h before infection with 100 LD50 of HTNV (76–118 strain). Mice were weighed individually every day. The survival rates of HTNV infected mice were calculated daily, and the experiment was terminated on day 28. Statistical analysis using the log rank test determined the increase in survival rate with arbidol treatment to be significant (P<0.05). One-way ANOVA was used to determine statistical differences for MTD. bP<0.05 vs placebo-treated controls.
Effect of arbidol on histopathological changes
Four animals from each group were sacrificed on day 12 pi, and the survivors were sacrificed on day 28 pi. The lungs, brains, and kidneys were collected for pathological examination. The organs of normal controls did not exhibit any modification in their structure (see Figure 2D). However, all infected animals developed histopathological changes with various degree of severity on day 12 pi. The lesions were as follows (Figure 2A): brain (scattered hemorrhages, congestion, edema, and focal necrosis), lung (thickened alveolar walls, interstitial lymphocyte and macrophage infiltration, and hemorrhage), and kidney (focal renal interstitial hemorrhage, congestion). On day 12 pi, the lungs of arbidol-treated animals (10 and 20 mg·kg−1·d−1) demonstrated a significant reduction in histopathological changes, and the brains and kidneys showed decreased edema and focal hemorrhage compared to placebo controls (Figure 2B). On day 28 pi, no histological changes were detected in the lungs, kidneys and brains of survivors (Figure 2C).
Effects of arbidol on organ histopathology in suckling mice infected with HTNV (×400). On day 12 pi and 28 pi four mice were sacrificed to prepare sections for H&E stain. (a1−a3) Histological section from a placebo controls animal (arrow in upper panel). (a1) Lung (thickened alveolar walls, interstitial lymphocyte and macrophage infiltration, and hemorrhage). (a2) Kidney (focal renal interstitial hemorrhage, congestion). (a3). Brain (focal necrosis and hemorrhage). (b1−b3) Histological section from one of the mice treated with arbidol at 20 mg·kg−1·d−1 on day 12 pi. (c1–c3) Histological section from one of the mice treated with arbidol at 20 mg·kg−1·d−1 on day 28 pi. (d1–d3) Histological section from a normal control. Scale bar=25 μm.
Inhibition of viral antigen expressed in the organs by arbidol
We detected viral antigen in the lungs, brains, and kidneys of animals using IFA as described above. The organs of normal controls did not exhibit immunofluorescence (Figure 3D). In comparison, specific fluorescence was observed in the lungs, brains, and kidneys of placebo controls (Figure 3A). At day 12 pi, the lungs of arbidol-treated animals (10 and 20 mg·kg−1·d−1) demonstrated a significant reduction in immunofluorescence, and a reduction in viral antigen was expressed in the brains and kidneys compared to placebo-treated controls (Figure 3B). On day 28 pi, viral antigen was not detected in the brains of survivors (Figure 3C). Lungs and kidneys showed much less immunofluorescence (Figure 3C).
Inhibitory effects of arbidol on viral antigen expressed in organs of suckling mice infected with HTNV (×400). On day 12 and 28 pi, four mice from each group were sacrificed and sections prepared for IFA. (a1–a3) On day 12 pi viral antigen was detected in the organs (arrow) of a placebo-treated animal. (a1) Lung; (a2) Kidney; (a3) Brain; (b1–b3) Viral antigen was detected in the animal treated at 20 mg·kg−1·d−1 arbidol on day 12 pi. (c1–c3) Viral antigen expressed in a survival animal at 28 dpi. (d1–d4) A mock-infected animal. Scale bar=25 μm.
Inhibition of HTNV mRNA by arbidol
Semi-quantitative RT-PCR analysis was performed on total RNA extracted from tissues of infected animals as described above in “Materials and Methods.” No viral mRNA was detected on day 4 pi in the lungs, kidneys, and brains of mice infected by HTNV (Figure 4A). The mRNA/β-actin ratio in the lungs decreased in a dose- and time-dependent manner. On day 16 pi, no viral mRNA was detected in the lungs of animals treated with arbidol at 10 or 20 mg·kg−1·d−1, and the ratio in the brains decreased in a dose-dependent manner. Animals treated with arbidol at 20 mg·kg−1·d−1 showed an mRNA/β-actin ratio in the kidneys of 0.24 (P<0.05), whereas the ratio of placebo controls was 0.88 (Figure 4B). To further investigate the effect of arbidol on HTNV gene expression, the viral mRNA in the tissues of survivors (day 28 pi) was measured. After treatment with arbidol at 10 or 20 mg·kg−1·d−1, no viral mRNA was detected in the lungs or brains, but viral mRNA was detected in the kidneys.
Inhibitory effects of arbidol on viral load in organs of suckling mice infected with HTNV (n=4). On day 4, 8, 12, and 16 pi, viral loads in the lungs, kidneys and brains were determined by semi-quantitative RT-PCR and plaque assay as described in “Materials and Methods.” Each data point represents the mean from three independent experiments. (a) Images of RT-PCR. The S segment of the viral genome (242 bp) was chosen for detection. M: DNA marker; NC: Normal controls; HD: 20 mg·kg−1·d−1; MD: 10 mg·kg−1·d−1; LD: 5 mg·kg−1·d−1; VC: Placebo controls; (b) Relative expression of viral S gene mRNA against β-actin in HTNV infected mice. (b1) Lung; (b2) Kidney; (b3) Brain; bP<0.05 vs placebo-treated controls. eP<0.05 vs HD. (c) Titers of infectious viral particles in the organs were determined by the standard plaque assay. (c1) Lung; (c2) Kidney; (c3) Brain.
Reduction of HTNV titers by arbidol
The titers of infectious viral particles were determined by the standard plaque formation assay described above. No infectious viral particles were detected on day 4 pi in the tissues of animals infected by HTNV. As shown in Figure 4C, viral titers in the lungs of animals treated with arbidol were reduced in a dose- and time-dependent manner in comparison to placebo-treated controls. After treatment with arbidol at 20 mg·kg−1·d−1, viral titers in the kidneys showed a significant reduction at day 16 pi. The mean viral yields of the brains decreased in a dose-dependent manner at day 16 pi. No infectious viral particles were detected in the lungs, kidneys, and brains of survivors (day 28 pi).
Effect of arbidol on serum TNF-α levels of HTNV-infected mice
The concentrations of serum TNF-α were tested by ELISA to determine the immunologic effect of arbidol on mice infected with HTNV. At day 4 pi, the serum TNF-α level in normal animals was 79.56 pg/mL. In comparison, the serum TNF-α concentration in placebo controls was 183.58 pg/mL (P<0.05). Compared with placebo controls, treatment with arbidol decreased secretion of TNF-α in a dose-dependent manner (P<0.05). On days 8, 12, and 16 pi, serum TNF-α levels in animals treated with arbidol doses of 10 and 20 mg·kg−1·d−1 were between 30–80 pg/mL, while those of placebo controls were below the limit of detection (15.63 pg/mL). The results are shown in Table 2.
Discussion
Antiviral activity of arbidol against HTNV in vitro
In the present study, arbidol inhibited HTNV replication when added before or after infection, with an IC50 of 0.9 and 1.2 μg/mL and an SI of 17.4 and 13, respectively, indicating that arbidol is highly active against HTNV. It is unknown why no virucidal effect of arbidol was observed in this experiment whereas virucidal effect was exhibited for some other viruses15. It is possible that the arbidol concentration evaluated is below the IC50. Vero cells are characterized by a chromosomal defect that can block type I interferon (IFN) expression21. Our data suggest that arbidol can induce durable antiviral activity in Vero cells, not only blocking the adsorption of viral particles onto cells, but also inhibiting the late stage of the viral replication cycle. Therefore, the antiviral activity of arbidol may not be completely due to IFN production, but, rather, related to the effect of arbidol on altering cellular microenvironments to restrain HTNV amplification. Previous reports16 demonstrated that arbidol's affinity for membranes may inhibit several aspects of the HCV life cycle, and we propose that a similar action mechanism may exist during the inhibition of HTNV infection.
Safety of arbidol for suckling BALB/c mice
A previous study showed that the LD50 dose for female BALB/c mice with oral gavage treatment was approximately 314 mg·kg−1·d1. We demonstrated that the LD50 dose for suckling BALB/c mice orally administered arbidol for 10 days was 78.42 mg·kg−1·d−1. Furthermore we found that suckling mice infected by HTNV and treated with arbidol at 40 mg·kg−1·d−1 showed pronounced toxicity, as evidenced by weight loss, with death occurring 7 or 8 dpi (data not shown). Therefore, the testable doses we chose were 5, 10, and 20 mg·kg−1·d−1.
Establishing a newborn BALB/c mouse model for HTNV infection
A number of animal models for the study of HFRS exist22, 23, but lethal disease could be induced only in newborn and immunodeficient animals24, 25, 26. Thus, we established a model of infection by ic inoculation of suckling mice with HTNV as used before20. Infected animals showed characteristic progression of disease findings and lesions caused by the infection, as well as virological parameters detected in the target organs. Moreover, the negative histopathological test results of mock-infected animals (Figure 2) excluded the possibility that the inoculation route could have been directly or indirectly responsible for the lethal disease. All these findings together suggest that suckling BALB/c mice infected with HTNV can be used as a suitable model to evaluate antiviral agents.
Arbidol is effective against HTNV in vivo
The route of administration of compounds is important in vivo, and arbidol is rather insoluble in aqueous medium. Therefore, oral gavage was used for administration of arbidol in the study. Based on other authors' reports15 and our results in vitro, treatment of the animals was initiated 24 h before infection. We demonstrated that orally administered arbidol at 10 or 20 mg·kg−1·d−1 significantly reduced the appearance of clinical signs of HTNV and increased the survival rate and MTD (Figure 1). By day 28, all survivors were asymptomatic, demonstrated weight gain, and exhibited no histopathological changes in their organs. The expression of viral antigen significantly decreased in the lungs and kidneys, and no HTNV antigen was detected in the brains of survivors. All these findings suggest that arbidol has the ability to elicit protective antiviral activity against HTNV in vivo, with a minimum effective concentration of 10 mg·kg−1·d−1 as demonstrated in this experiment. Semi-quantitative RT-PCR data showed that the highest viral replication levels in the lungs, kidneys, and brains of placebo controls appeared at day 16 pi, which is similar to others' results25, 27, and that animals treated with arbidol showed lower peak levels and/or a delay in reaching peak levels (Figure 4B). Viral titers in the lungs first decreased in a dose- and time-dependent manner, followed by decreases in the brains and then in the kidneys (Figure 4C), as determined by plaque assay, indicating that treatment with arbidol reduces infectious virus as well as total viral RNA in the organs. After treatment with arbidol, no viral mRNA or infectious viral particles were detected in the lungs and brains of survivors. Although viral RNA was demonstrated in the kidneys of all arbidol-treated mice that survived HTNV infection, no infectious viral particles were observed in any of the mice, indicating that the major target tissues of HTNV in suckling mice are the kidneys, but HTNV failed to replicate in the presence of arbidol. Taken together, these results suggest that arbidol can inhibit HTNV replication in vivo, with the difference in efficacy in different organs perhaps being due to the tissue distribution of arbidol and the organization tropism of HTNV. We demonstrated that no viral mRNA or infectious viral particles in tissues of animals infected by HTNV was detected on day 4 pi, suggesting a very low level of virus replication in the organs. This is in agreement with the results of Kim et al25.
It has been reported that increased plasma levels of TNF-α were found in the blood of HFRS patients during the acute phase28. The results of Niikura et al29 confirmed that HTNV infection of endothelial cells might contribute to increased vascular leakage through a prolonged response to TNF-α. In our investigations, serum TNF-α levels were higher on day 4 pi, showing that treatment with arbidol can decrease levels of TNF-α on day 4 pi in a dose-dependent manner (Table 2). Arbidol has been shown to have effects on activating macrophages14, suggesting that the effect of arbidol on modulating TNF-α level may be due to activating macrophages. Serum levels of TNF-α showed a decrease at days 8, 12, and 16 pi, which may be due to secreted TNF-α binding to TNF-α receptors (TNFR) expressed in the cell membrane or secreted in serum30, 31, 32; thus, resulting in less detection of secreted TNF-α. Higher levels of TNF-α were detected in arbidol-treated animals than placebo controls, suggesting that the mechanism of action of arbidol may not only involve modulating serum TNF-α levels but also expression of TNFR in the tissues32, 33, 34. Therefore, it is possible that apart from exhibiting anti-HTNV activity, arbidol could have also modulated the TNF responses and protected mice against the lethal challenge.
In conclusion, arbidol has the ability to elicit protective antiviral activity against HTNV in vivo and in vitro. Arbidol was found to present potent inhibitory activity against HTNV when added before or after viral infection in vitro. Orally administered arbidol can reduce histopathological changes, decrease expression of viral antigen and viral load, and modulate the TNF responses. Further experiments are needed to investigate the mechanism of action of arbidol. Arbidol may be a promising candidate for early treatment of Hantavirus infection.
Author contribution
Hai-ying DENG performed research, analyzed data, and wrote the paper; Fan LUO performed research and analyzed data; Li-qiao SHI analyzed data; Qiong ZHONG and Ying-juan LIU performed research; Zhan-qiu YANG designed the study, analyzed data, and wrote the manuscript.
References
Kruger DH, Urich R, Lundkvist A . Hantavirus infections and their prevention. Microbes Infect 2001; 3: 1129–44.
Muranyi W, Bahr U, Zeier M, van der Woude FJ . Hantavirus infection. J Am Soc Nephrol 2005; 16: 3669–79.
Lee HW . Epidemiology and pathogenesis of hemorrhagic fever with renal syndrome. In: Elliott RM, editor. The Bunyaviridae. New York (NY): Plenum Press; 1996. p253–67.
Schmaljohn C, Hjelle B . Hantaviruses-A global disease problem. Emerg Infect Dis 1997; 3: 95–104.
Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, et al. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA 2002; 287: 2391–405.
Kanerva M, Mustonen J, Vaheri A . Pathogenesis of Puumala and other Hantavirus infections. Rev Med Virol 1998; 8: 95–104.
Klempa B, Stanko M, Labuda M, Ulrich R, Meisel H, Kruger DH . Central European Dobrava Hantavirus isolate from a striped field mouse (Apodemus agrarius). J Clin Microbiol 2005; 43: 2756–63.
Hooper J, Li D . Vaccines against Hantaviruses. Curr Top Microbiol Immunol 2001; 256: 171–91.
Robert W Sidwell, Smee DF . Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res 2003; 57: 101–11.
Huggins JW, Hsiang CM, Cosgriff TM, Guang MY, Smith JI, Wu ZO, et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis 1991; 164: 1119–27.
Chapman LE, Mertz GJ, Peters CJ . Intravenous ribavirin for hantavirus pulmonary syndrome: safety and tolerance during 1 year of open-label experience. Ribavirin Study Group. Antiviral Ther 1999; 4: 211–9.
Salmon-Céron D, Chauvelot-Moachon L, Abad S, Silbermann B, Sogni P . Mitochondrial toxic effects and ribavirin. Lancet 2001; 357: 1803–4.
Ohnson EM . Developmental toxicity and safety evaluations of ribavirin. Pediatr J Infect Dis 1990; 9: S85–7.
Glushkov RG, Gus'kova TA, Krylova LI, Nikolaeva IS . Mechanisms of arbidole's immunomodulating action. Vestn Ross Akad Med Nauk 1999; 3: 36–40. In Russian.
Shi LQ, Xiong HR, He J, Deng HY, Li Q, Zhong Q, et al. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol 2007; 152: 1447–55.
Boriskin YS, Pecheur EI, Polyak SJ . Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection. Virol J 2006; 3: 56.
Brooks MJ, Sasadeusz JJ, Tannock GA . Antiviral chemotherapeutic agents against respiratory viruses: where are we now and what's in pipeline? Curr Opin Pulm Med 2004; 10: 197–203.
Denizot F, Lang R . Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986; 89: 271–7.
Severson WE, Schmaljohn CS, Javadian A, Jonsson CB . Ribavirin causes error catastrophe during Hantaan virus replication. J Virol 2003; 77: 481–8.
Liu YJ, Yang ZQ, Deng HY, Xiao H, Qu CF . Separation and anti-hantaan virus activity of extracts from alternanthera philoxcroides in vitro and in vivo. J Wuhan Univ (Nat Sci) 2007; 12: 1143–7.
Diaz MO, Ziemin S, Lebeau MM, Pitha P, Smith SD, Chilcote RR, et al. Homozygous deletion of the alpha-interferon and beta-1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci USA 1988; 85: 5259–63.
Lee PW, Amyx HL, Gibbs CJ Jr, Gajdusek DC, Lee HW . Propagation of Korean hemorrhagic fever virus in laboratory rats. Infect Immun 1981; 31: 334–8.
Yoshimatsu K, Arikawa J, Ohbora S, Itakura C . Hantavirus infection in SCID mice. J Vet Med Sci 1997; 59: 863–8.
Brian BG, Michael RH . Animal models of highly pathogenic RNA viral infections: Hemorrhagic fever viruses. Antiviral Res 2008; 78: 79–90.
Gum RK, Kelly T, Mckee JR . Pathogenesis of Hantaan virus infection in suckling mice: clinical, virologic, and serologic observations. Am J Trop Med Hyg 1985; 34: 388–95.
Yamanouchi T, Domae K, Tanishita O, Takahashi Y, Yamanishi K, Takahashi M, et al. Experimental infection in newborn mice and rats by hemorrhagic fever with renal syndrome (HFRS) virus. Microbiol Immunol 1984; 28: 1345–53.
Huggins JW, Kim GR, Brand OM, McKee KT Jr . Ribavirin therapy for Hantaan virus infection in suckling mice. J Infect Dis 1986; 153: 489–97.
Linderholm M, Ahlm C, Settergren B, Waage A, Tarnvik A . Elevated plasma levels of tumor necrosis factor (TNF)–alpha soluble TNF receptors interleukin (IL)-6 and IL-10 in patients with hemorrhagic fever with renal syndrome. J Infect Dis 1996; 173: 38–43.
Niikura M, Maeda A, Ikegami T, Saijo M, Kurane I, Morikawa S . Modification of endothelial cell functions by Hantaan virus infection: prolonged hyper-permeability induced by TNF-alpha of Hantaan virus-infected endothelial cell monolayers. Arch Virol 2004; 149: 1279–92.
Wei XW, Gong JF, Zhu J, Wang PF, Li N, Zhu WM, et al. The suppressive effect of triptolide on chronic colitis and TNF-α/TNFR2 signal pathway in interleukin-10 deficient mice. Clin Immunol 2008; 129: 211–8.
Strandin T, Hepojoki J, Wang H, Vaheri A, Lankinen H . Hantaviruses and TNF-alpha act synergistically to induce ERK1/2 inactivation in Vero E6 cells. Virol J 2008; 5: 110.
Wajant H, Pfizenmaier K, Scheurich P . Tumor necrosis factor signaling. Cell Death Differ 2003; 10: 45–65.
Krogerus L, Soots A, Loginov R, Bruggeman C, Lautenschlager I . CMV increases tubular apoptosis through the TNF-α-TNF-R1 pathway in a rat model of chronic renal allograft rejection. Transplant Immunol 2008; 18: 232–6.
Temonen M, Lankinen H, Vapalahti L, Ronni T, Julkunen I, Vaheri A . Effect of interferon-α and cell differentiation on Puumala virus infection in human monocyte/macrophages. Virology 1995; 206: 8–15.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (NSFC project No 30873104).
We thank Dr Rhea-Beth MARKOWITZ and Dr Pamela Wall STEEN from the Medical College of Georgia, Augusta, Georgia, USA for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, Hy., Luo, F., Shi, Lq. et al. Efficacy of arbidol on lethal hantaan virus infections in suckling mice and in vitro. Acta Pharmacol Sin 30, 1015–1024 (2009). https://doi.org/10.1038/aps.2009.53
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2009.53
Keywords
This article is cited by
-
Multicenter, randomized controlled, open label evaluation of the efficacy and safety of arbidol hydrochloride tablets in the treatment of influenza-like cases
BMC Infectious Diseases (2023)
-
IFN-λs inhibit Hantaan virus infection through the JAK-STAT pathway and expression of Mx2 protein
Genes & Immunity (2019)
-
The Antiviral Drug Arbidol Inhibits Zika Virus
Scientific Reports (2018)
-
Specific interference shRNA-expressing plasmids inhibit Hantaan virus infection in vitro and in vivo
Acta Pharmacologica Sinica (2016)
-
The Location of the Protonated and Unprotonated Forms of Arbidol in the Membrane: A Molecular Dynamics Study
The Journal of Membrane Biology (2016)