Abstract
Aim:
The aim of this study was to creatively implement a novel chemo-gene-virotherapeutic strategy and further strengthen the antitumor effect in cancer cells by the combined use of ZD55-IL-24 and cisplatin.
Methods:
ZD55-IL-24 is an oncolytic adenovirus that harbors interleukin 24 (IL-24), which has a strong antitumor effect and was identified and evaluated by PCR, RT-PCR, and Western blot analysis. Enhancement of cancer cell death using a combination of ZD55-IL-24 and cisplatin was assessed in several cancer cell lines by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and cytopathic effect (CPE) assay. Apoptosis induction by treatment with ZD55-IL-24 and/or cisplatin was detected in BEL7404 and SMMC7721 by morphological evaluation, apoptotic cell staining, and flow cytometry analysis. In addition, negative effects on normal cells were evaluated in the L-02 cell line using the MTT assay, the CPE assay, morphological evaluation, apoptotic cell staining, and flow cytometry analysis.
Results:
The combination of ZD55-IL-24 and cisplatin, which is superior to ZD55-IL-24, cisplatin, and ZD55-EGFP, as well as ZD55-EGFP plus cisplatin, resulted in a significantly increased effect. Most importantly, conjugation of ZD55-IL-24 with cisplatin had toxic effects equal to that of cisplatin and did not have overlapping toxicities in normal cells.
Conclusion:
This study showed that ZD55-IL-24 conjugated with cisplatin exhibited a remarkably increased cytotoxic and apoptosis-inducing effect in cancer cells and significantly reduced the toxicity in normal cells through the use of a reduced dose.
Similar content being viewed by others
Introduction
MDA-7/IL-24 (melanoma differentiation associated gene-7/interleukin-24), which was first classified as a member of the interleukin (IL)-10 gene family, has attracted particular attention1 because it can specifically induce apoptosis in a wide variety of malignant cells but spares normal cells2, 3. Many studies proved that IL-24 exhibited potent “bystander antitumor” activity4 and enhanced radiation lethality5, induced immune-regulatory activities6, and inhibited tumor angiogenesis7. In addition, Ad.IL-24, which is a recombinant adenovirus that encodes the human IL-24 gene, has been evaluated in a phase I/II clinical trial in patients with advanced carcinoma8, 9, which showed that IL-24 was a potent therapeutic gene for human cancers. Recently, the novel oncolytic adenovirus vector, ZD55, was constructed by deleting an E1B 55-kDa gene of adenovirus 5. ZD55 is similar to the virotherapy agent, ONYX-015, which is an oncolytic adenovirus with a deleted E1B 55-KDa that selectively replicates in p53-deficient tumor cells and lyses them. The novel gene-virotherapy strategy for cancer using the delivery of therapeutic genes by ZD55 was first reported by us and indicated a strong antitumor effect both in vitro and in vivo10, 11. We have previously demonstrated that ZD55-IL-24 exhibits a strong tumor suppression effect in a panel of tumor cells and has a high antitumor effect for human colorectal carcinoma xenografts in nude mice12. Furthermore, ZD55-IL-24 has been exploited as an anticancer drug in China for its potent antitumor activity.
Chemotherapy is one of the most conventional therapeutic strategies for human cancers. Cisplatin, which is also named cis diamminedichloroplatinum (DDP), is deemed to be the “penicillin of cancer drugs” due to its universal, early, and effective treatment for many cancers13. In fact, cisplatin is often used as part of an attractive chemotherapy regimen and is widely used to treat a variety of cancers, including ovarian, head and neck, bladder, prostate, cervical, testicular, lung, gullet, stomach, and other neoplasms. To date, the mechanism has not yet been fully elucidated, and cisplatin is generally believed to kill cancer cells by binding to DNA and interfering with the cell's repair mechanism, which eventually leads to cell death14. Unlike many anticancer drugs, which are organic molecules that have complex structures, cisplatin is an inorganic molecule with a simple structure. Despite these merits, severe toxic side effects and drug resistance are major clinical obstacles associated with cisplatin therapy15, 16. The dose that is necessary to overcome even a small increase in cellular resistance can result in severe cytotoxicity in normal cells. Therefore, it is urgent to explore novel approaches to reduce drug dosage, minimize side effects, enhance the efficacy of therapy, and promote the application of cisplatin in cancer therapy.
Although either ZD55-IL-24 or cisplatin alone displays potent antitumor activity, some disadvantages exist, such as drug resistance and damage to normal cells. Thus, further investigation is required to increase the antitumor effects of these drugs. Chemo-gene-virotherapy, a novel strategy that combines a chemotherapeutic reagent, therapeutic genes, and an oncolytic virus (ZD55), was first proposed by us17. Previous reports have demonstrated that the combination of ZD55, carrying the TRAIL or Smac gene, and chemotherapeutic drugs significantly improved the tumor-killing effect and reduced side effects17, 18.
In this study, we utilized the above strategy and combined cisplatin with ZD55-IL-24 to investigate antitumor efficacy. Our data proved that conjugation of cisplatin with ZD55-IL-24 resulted in robust cytotoxicity in tumor cell lines without any overlapping toxicity in normal cells. In addition, negative effects were avoided by the use of the tumor specific-replication adenovirus and the decreased drug dose. This is the first study in which ZD55-IL-24 was applied in a novel chemo-gene-virotherapy strategy and it demonstrated the potential of gene therapy for human cancers.
Materials and methods
Cells and cell culture
HEK293 (human embryonic kidney cell line containing the E1A region of adenovirus) was obtained from Microbix Biosystems, Inc (Toronto, Ontario, Canada). L-02 (normal human liver cell line), BEL7404 (human hepatocellular carcinoma cell line, HCC), SMMC7721 (HCC cell line), H1299 (human lung adenocarcinoma cell line), HCT116 (human colorectal cancer cell line), HeLa (human cervical cancer cell line), and CNE (human nasopharyngeal carcinoma cell line) were purchased from the Shanghai Cell Collection (Shanghai, China). The HEK293 cell line was cultured in Dulbecco's modified Eagle's medium (DMEM; GIBCOBRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS; GIBCOBRL). Other cell lines were cultured in DMEM supplemented with 5% heat-inactivated FBS. All cell lines were cultured at 37 °C in a 5% CO2 humidified incubator.
Plasmids and generation of recombinant adenovirus
The plasmids, pZD55-EGFP and pZD55-IL-24, were previously constructed in our laboratory. The oncolytic adenoviruses, ZD55-EGFP and ZD55-IL-24, were generated in HEK293 cells by homologous recombination between pZD55-EGFP or pZD55-IL-24 and the adenovirus packaging plasmid, pBHGE3 (Microbix Biosystems, Toronto, Canada), respectively12. ZD55-EGFP and ZD55-IL-24 were amplified in HEK293 cells, purified by cesium chloride gradient ultracentrifugation and subjected to dialysis. Virus titer was measured using a standard plaque formation assay in HEK293 cells.
Identification of recombinant viruses by conventional PCR
The viral genome was extracted from the purified stock of ZD55-EGFP or ZD55-IL-24 using a QIAamp DNA blood mini kit (QIAGEN, Germany). The EGFP gene and IL-24 gene were verified by conventional PCR using the following primers (EGFP gene forward: 5′-AGCTGGACGGCGACGTAAAC-3′ and reverse: 5′-CACGAACTCCAGCAGGACATG-3′; IL-24 gene forward: 5′-GAATTCGATATCTCTAGA C-3′ and reverse: 5′-ATAGATATCTCAGAGCTTGTA-3′). The cycle conditions were 94 °C for 5 min, followed by 30 cycles at 94 °C for 1 min, 50 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 10 min. The amplification product was visualized by electrophoresis on a 1% agarose gel containing ethidium bromide.
RT-PCR analysis
Total cellular RNA was isolated using TRIzol reagent (Life Technologies, USA) according to the manufacturer's protocol. Reverse-transcription (RT)-PCR was performed with total RNA (2 μg) using the First Strand RT-PCR Kit (Stratagene, USA). A cDNA equivalent of 1 ng RNA was amplified by PCR using primers specific for the target gene. The forward and reverse primers for the IL-24 gene (649 bp) were 5′-GAATTCGATATCTCTAGAC-3′ and 5′-ATAGATATCTCAGAGCTTGTA-3′, respectively. The cycle conditions were the same as above. The amplified products were visualized by electrophoresis on a 1% agarose gel containing ethidium bromide. In addition, a control PCR for the detection of DNA contamination was performed using RNA samples devoid of the RT enzyme. The results were negative in all RNA samples, which indicated that our RNA preparations were not contaminated by viral DNA.
Western blot analysis
Cells were harvested from the plates and resuspended in lysis buffer. Protein concentrations were determined with the Bio-Rad protein assay system. Total proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on an 8%–12% gel and then transferred to a 0.45-μm nitrocellulose membrane (Millipore, USA). The membrane was blocked with blocking buffer (5% bovine serum albumin, 10 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, and 0.05% Tween 20) overnight at 4 °C, and then incubated with primary antibodies (1:1000 dilutions). After incubation in the dark with IR Dye 700 or IR Dye 800 conjugated IgG secondary antibodies (1:4000 dilution, Rockland Inc, UK), immunodetection was performed using the Odyssey Infrared Imaging System (LI-COR Biosciences, USA). The primary antibodies of actin, E1A, and poly (ADP-ribose) polymerase (PARP) were purchased from Santa Cruz Biotechnology (Santa Cruz, USA). The IL-24 primary antibody was obtained from GenHunter (Nashville, TN).
Cell viability assay
Cells were plated in a 96-well plate and treated with the adenovirus, cisplatin (Alexis, Switzerland), or a combination of the virus and cisplation at the indicated MOI (Multiplicity of Infection, ratio of infectious virus particles to cells) or drug dosage. At the indicated time, the medium was removed and fresh medium containing 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT, 0.5 mg/mL, Sigma) was added to each well. Cells were incubated at 37 °C for 4 h. The supernatant was removed, and 150 μL DMSO was subsequently added to each well and mixed thoroughly. Absorbance was read at 595 nm with a Bio-Rad microplate reader (Hercules, CA).
Cytopathic effect (CPE) assay
Human hepatocellular carcinoma cell lines (BEL7404 and SMMC7721), human cervical cancer cell line (HeLa), and normal liver cell line (L-02) were plated in a 96- or a 48-well plate and treated with cisplatin alone, the virus alone, or a combination of cisplatin and the virus at the indicated dosage. 72 or 120 h after treatment, the cells were washed, paraformaldehyde-fixed, and stained with crystal violet (Amresco, USA).
Flow cytometry analysis Cells were plated on 6-well plates at a certain density and treated with cisplatin alone, virus alone, or a combination of cisplatin and the virus at the indicated dosage. Cells were harvested 48 h after infection, trypsinized, and washed once with complete medium. Aliquots of cells (5×105) were resuspended in 500 μL of binding buffer and stained with fluorescein isothiocyanate (FITC)-labeled annexin V (BioVision, Palo Alto, CA). A fluorescence-activated cell-sorting (FACS; Becton Dickinson) assay was performed immediately after staining.
Morphological evaluation
Cells were plated in a 48-well plate overnight and then treated with ZD55-EGFP and/or cisplatin or ZD55-IL-24 and/or cisplatin, and analyzed by microscopy for signs of apoptosis or cell death. The cells were examined 24 h and 48 h after treatment, respectively.
Apoptotic cell staining
Cells seeded in 96-well plates were treated with ZD55-EGFP, ZD55-IL-24, cisplatin, ZD55-EGFP plus cisplatin, ZD55-IL-24 plus cisplatin, or phosphate-buffered saline (PBS). After 48 h or 72 h of treatment, the cells were incubated with Hoechst 33342 (Molecular Probes, Eugene, OR) for 30 min, washed with PBS twice, and observed under a fluorescence microscope.
Statistical analysis
All data are displayed as means±SD. Student's t-test was applied to analyze the relationship between the different variables. Statistical significance was assumed when P<0.05.
Results
Characterization and Identification of recombinant oncolytic viruses
The oncolytic adenovirus vector, ZD55, was constructed by deleting the E1B 55-kDa gene of adenovirus 5, which can selectively replicate and lyse in a large number of tumor cells. Based on the novel gene-virotherapy strategy, we utilized ZD55 to deliver the therapeutic gene IL-24 under the control of the human CMV-IE promoter and obtained the recombinant oncolytic adenovirus, ZD55-IL-24. The control virus, ZD55-EGFP, was constructed using the same methods. The packaging and purification of recombinant virus were performed as previously described21, and the titer of ZD55-IL-24 and ZD55-EGFP contained 5×109 plaque-forming unit (pfu)/mL and 1×1010 pfu/mL, respectively. To identify the expected recombinant virus, the virus DNAs were extracted and PCR was performed to detect the corresponding exogenous gene (IL-24 and EGFP) of the recombinant virus. There is a distinct DNA fragment from the IL-24 (649 bp) and EGFP (623 bp) genes in each virus (Figure 1A, 1B).
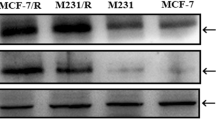
Identification of recombinant oncolytic adenovirus. (A) Identification of the IL-24 gene in ZD55-IL-24 by PCR. Lane 1: negative control; lane 2: positive control; lane M: DL2000 marker; lane 3: ZD55-IL-24. (B) Identification of EGFP in ZD55-EGFP by PCR. Lane 1: negative control; lane 2: positive control; lane M: DL2000 marker; lane 3: ZD55-EGFP. (C) The expression of IL-24 was detected by reverse transcription polymerase chain reaction (RT-PCR). Lane M: DL2000 marker; lane 1: pZD55-IL-24 (positive control); lane 2: ZD55-IL-24; lane 3: negative control. (D) Expression of E1A and IL-24 genes in L-02, BEL7404, and HeLa cells 48 h after infection of ZD55-EGFP.
To assess the transgenic delivery capability that is mediated by oncolytic adenovirus, ZD55, the HCC cell line BEL7404 was infected with ZD55-IL-24 for 48 h, and RT-PCR was performed to detect the expression of IL-24 gene. The results showed that IL-24 gene express efficiently in BEL7404 cells (Figure 1C). Furthermore, to prove the tumor-selective replication ability of the oncolytic adenovirus, we detected the expression of the adenovirus E1A protein and the therapeutic gene IL-24 by Western blot analysis in two tumor cell lines (BEL7404 and HeLa) and a normal cell line (L-02) that were infected with ZD55-EGFP and ZD55-IL-24. The strong expression of E1A in tumor cells indicated that ZD55-EGFP and ZD55-IL-24 could replicate at higher levels in tumor cells than in normal cells (Figure 1D). In addition, the expression of the IL-24 gene in tumor cells treated ZD55-IL-24 was observed at a much higher level than in normal cells. These results suggest that the oncolytic adenovirus ZD55 is tumor-specific and could significantly mediate efficient exogenous gene expression in tumor cells.
Enhanced antitumor effect using a combination of ZD55-IL-24 and cisplatin
As a conventional chemotherapeutic drug, cisplatin was reported to augment IL-24 or ONYX-015-mediated virotherapy in tumor cells from several origins19, 20. To evaluate the cytotoxic effects of ZD55-IL-24 and cisplatin, two HCC cell lines (BEL7404, SMMC7721), a human lung cancer cell line (H1299), a human colorectal cancer cell line (HCT116), a human cervical cancer cell line (HeLa), and a human nasopharyngeal carcinoma cell line (CNE) were infected with ZD55-IL-24 and ZD55-EGFP separately or in combination with cisplatin. The MTT assay was performed, and the results are shown in Figure 2 (P<0.05). The cell viability in the six tumor cell lines treated with a combination of ZD55-IL-24 (5 MOI) and cisplatin (3 μg/mL) decreased to approximately 30%-80% in a time-dependent manner compared with ZD55-EGFP (5 MOI) alone, ZD55-IL-24 (5 MOI) alone, cisplatin (3 μg/mL) alone, or ZD55-EGFP (5 MOI) plus cisplatin (3 μg/mL). About 81% of HeLa cells and 73% of SMMC7721 cells were killed by the combination of ZD55-IL-24 and cisplatin for 72 h, but such a phenomenon could not be observed with other treatments. In addition, ZD55-EGFP or ZD55-IL-24 in combination with cisplatin was significantly different from either treatment alone for the six kinds of tumor cells (P<0.05), which indicated that the combination of the oncolytic adenovirus with cisplatin had an obviously enhanced antitumor effect. Other than CNE cells, comparison of the other five tumor cells with regard to treatments using ZD55-IL-24 plus cisplatin and ZD55-EGFP plus cisplatin was significant (P<0.05) or very significant (P<0.01), which indicates that the IL-24 gene can induce tumor cell apoptosis and kill the cells. The results suggest that the combination of ZD55-IL-24 and cisplatin has an enhanced tumor-killing effect.
Enhanced suppression of tumor cell proliferation using the combination of ZD55-IL-24 and cisplatin. The tumor cells BEL7404 (A), SMMC7721 (B), H1299 (C), HCT116 (D), HeLa (E), and CNE (F) were treated with ZD55-EGFP (5 MOI), ZD55-IL-24 (5 MOI), cisplatin (3 μg/mL), ZD55-EGFP (5 MOI)+cisplatin (3 μg/mL), or ZD55-IL-24 (5 MOI)+cisplatin (3 μg/mL). Cell viability was determined by the MTT assay. Data are presented as means±SD of three independent experiments.
The cytotoxic effects of ZD55-IL-24 or cisplatin and the combination treatment on HeLa, SMMC7721, and BEL7404 were also assessed by crystal violet staining (Figure 3). When the cells were treated with a combination of ZD55-IL-24 and cisplatin, the enhanced cytotoxicity was significantly more potent than that of the other treatments.
The CPE assay was performed by crystal violet staining. The tumor cell lines BEL7404, SMMC7721, and HeLa were infected with ZD55-EGFP, ZD55-IL-24, cisplatin, a combination of ZD55-EGFP and cisplatin, or a combination of ZD55-IL-24 and cisplatin at the indicated doses. 120 h later, the cells were stained with crystal violet.
Apoptosis induction by treatment with ZD55-IL-24 and/or cisplatin
ZD55-IL-24 could efficiently mediate IL-24 expression in tumor cells, as shown in our previous study21. We detected IL-24 protein expression in HCC BEL7404 cells and human cervical cancer HeLa cells by Western blotting (Figure 1D). To determine whether cisplatin affects IL-24 expression, we treated the cancer cell line, BEL7404, with ZD55-IL-24 alone or ZD55-IL-24 plus cisplatin. The results indicated that cisplatin did not attenuate the expression of IL-24 (Figure 4). To determine the underlying mechanism by which ZD55-IL-24, cisplatin alone, or a combination of ZD55-IL-24 and cisplatin can induce apoptosis in cancer cells, the activation of death substrate poly (ADP-ribose) polymerase (PARP) was detected by Western blot analysis. The results showed that the cleavage of PARP (p85, the active form of PARP, which is typical of apoptosis) was observed in the tumor cells, BEL7404 and HeLa, after infection with ZD55-IL-24 for 48 h but not in the normal L-02 cells, which suggested that our oncolytic virus could cause tumor-specific apoptosis (Figure 5).
Flow cytometry analysis was performed to confirm the enhanced apoptosis of tumor cells after treatment with combined ZD55-IL-24 and cisplatin therapy. The percentage of apoptotic cells was determined by annexin V staining. The combination of ZD55-IL-24 with cisplatin showed a higher percentage of cell apoptosis in SMMC7721 and BEL7404 cells compared with other groups (Figure 6A). These observations were consistent with the morphological features. Most tumor cells died during treatment with combined ZD55-IL-24 and cisplatin, as shown by cell surface blebbing and the formation of apoptotic bodies (Figure 6B). Additionally, Hoechst 33342 staining also was performed to detect the apoptotic morphological changes of tumor cells after each treatment using a fluorescence microscope. More tumor cells treated with a combination of ZD55-IL-24 and cisplatin showed obvious apoptosis, including chromatin condensation and nuclear fragmentation (arrows indicate apoptotic cells), compared with other treatments (Figure 6B, 6C).
Detection of apoptosis and morphological evaluation of tumor cells. (A) Annexin V binding assay. The BEL7404 and SMMC7721 cells were treated with PBS, ZD55-EGFP, ZD55-IL-24, cisplatin, ZD55-EGFP plus cisplatin, or ZD55-IL-24 plus cisplatin at the indicated dose. 72 h later, the cells were harvested and stained with annexin V-FITC, which was immediately followed by flow cytometry analysis for apoptosis. The percentage of apoptotic cells was calculated using CellQuest software. Each value represents the mean of three wells. (B) BEL7404 cells and SMMC7721 cells were treated as above and the morphological changes of dead tumor cell or cells undergoing apoptosis were analyzed by microscopy after 72 h (Original magnification: ×200). (C) Cells were incubated with Hoechst 33342 for 30 min. and condensation and fragmentation of nuclei were observed under a fluorescence microscope (arrow) (Hoechst 33342 stain; original magnification: ×200).
Taken together, ZD55-IL-24 could lead to efficient tumor-specific viral replication and IL-24 expression and significantly induce tumor cell apoptosis. The combination of ZD55-IL-24 and cisplatin resulted in a significantly enhanced antitumor effect.
A dose-dependent manner of cytotoxicity in normal cells
To evaluate the safety of the cisplatin and virus combination, it is necessary to use a proper dosage that not only kills cancer cells but also has significantly reduced side effects on normal cells. According to the MTT data (P<0.05; Figure 7A), the lowest drug dosage of cytotoxicity was determined to be 5 MOI of ZD55-IL-24 and 3 μg/mL of cisplatin. Subsequent CPE assays, morphological evaluation, and apoptotic cell staining further confirmed that this dose of 5 MOI ZD55-IL-24 and 3 μg/mL cisplatin was reliable (Figure 7B, 7C, 7D). As a tumor-selective replication vector that drives a cancer specific pro-apoptotic gene, ZD55-IL-24 could theoretically spare normal cells. However, an extremely high dosage could still cause damage to normal cells (Figure 7A). Approximately 40% of L-02 cells were killed with ZD55-IL-24 treatment at 80 MOI. The dose-dependent effect of cisplatin (Figure 7A) demonstrated that more than 70% of L-02 cells were killed at the highest concentration (48 μg/mL) but only about 10% of normal cells died at the lowest concentration (3 μg/mL). Similarly, the combination of ZD55-IL-24 and cisplatin also resulted in dose-dependent cell death. Most importantly, the combination of ZD55-IL-24 and cisplatin had approximatively equal toxic effects compared with cisplatin alone but did not have any overlapping toxicity in normal cells.
Dose-dependent manner of toxicity to normal cells. (A) The MTT assay was used to determine the viability of L-02 cells at various doses of ZD55-IL-24, ZD55-IL-24 +cisplatin, or cisplatin after 48 h of treatment. Data are presented as means ± SD of three independent experiments. (B) CPE assay. Normal L-02 cells were treated with ZD55-IL-24, ZD55-IL-24+cisplatin, or cisplatin at the indicated doses. 72 h later, the cells were stained with crystal violet. (C) Morphological evaluation. L-02 cells were treated with ZD55-IL-24, ZD55-IL-24+cisplatin, or cisplatin and cell death was analyzed by microscopy after 24 h and 48 h. (Original magnification: ×100) (D) Apoptotic cell staining. Normal L-02 cells were treated with ZD55-IL-24 plus cisplatin at the indicated dose. Two days later, the cells were incubated with Hoechst 33342 and the condensation and fragmentation of nuclei was observed under a fluorescence microscope (arrow) (Hoechst 33342 stain; original magnification: ×200).
Morphological evaluation showed that most L-02 cells that were treated with ZD55-IL-24 (80 MOI) and cisplatin (48 μg/mL) were killed after 24 h, a trend that became more obvious after 48 h (Figure 7C). However, the L-02 cells treated with ZD55-IL-24 (5 MOI) and cisplatin (3 μg/mL) exhibited no apparent apoptosis, similar to that observed in the CPE analysis (Figure 7B). In addition, apoptotic cell staining clearly indicated that higher doses resulted in remarkable chromatin condensation and nuclear fragmentation in L-02 cells (Figure 7D).
To validate whether the combinatorial treatment with 3 μg/mL cisplatin and 5 MOI ZD55-IL-24 affected the survival of normal cells, an MTT assay was performed to evaluate the cell viability. The survival of L-02 cells was seldom affected by the combinatorial treatment (Figure 8A). The toxic effect of the conjugation group is similar to that of cisplatin, which showed that it was safe to use in normal cells. The annexin V binding assay also indicated that the combination of oncolytic virus and cisplatin did not lead to increased cell apoptosis compared with the individual treatments (Figure 8B). The results indicated that the above combination treatment was a potent anticancer therapy strategy and hardly affected the viability of normal cells.
Combinatorial treatment with cisplatin and ZD55-IL-24 did not significantly affect the survival of normal cells. (A) L-02 cells were treated with ZD55-EGFP (5 MOI), ZD55-IL-24 (5 MOI), cisplatin (3 μg/mL), ZD55-EGFP (5 MOI)+cisplatin (3 μg/mL), or ZD55-IL-24 (5 MOI)+cisplatin (3 μg/mL). Cell viability was determined by the MTT assay. Data are presented as means±SD of three independent experiments. (B) Apoptosis detection by annexin V binding assay. The L-02 cells were treated as in Figure 8A. 72 h later, the cells were harvested and stained with annexin V-FITC, which was immediately followed by flow cytometry. The percentage of apoptotic cells was calculated using CellQuest software. Each value represents the mean of 3 wells.
Discussion
MDA-7/IL-24 is a novel cancer growth-suppressing and apoptosis-inducing gene acquired by subtraction hybridization using a human melanoma cell line1. Some studies have indicated that increased expression of the IL-24 gene suppresses cell growth and induces cell apoptosis in a variety of cancer cells with single or multiple genetic defects, including alterations in p53, p16/INK4a, and Rb2, 21. The IL-24 gene is currently widely utilized in cancer gene therapy. Adenovirus mediated IL-24 gene overexpression in tumor cells is a promising way to improve cancer therapy. In our study, an E1B 55 kDa-deleted oncolytic adenovirus vector (ZD55) was engineered to specifically replicate and cause significant cytotoxic effects in all tested tumor cells while exhibiting low side effects in normal cells10, 22. Using the gene-virotherapy strategy, the ZD55-mediated IL-24 gene (ZD55-IL-24) exhibited potent antitumor activity and may represent a novel approach to cancer therapy.
Cisplatin is an inorganic compound that is broadly used for the treatment of various forms of malignant tumors, in particular head and neck cancer and several pediatric malignancies. However, these so-called virtues are often offset by disadvantages, including chemoresistance and toxicity23, 24. To eliminate drug resistance and reduce side effects, several conjugated strategies have been applied successfully in cancer therapy, such as the combination of cisplatin with the anticancer gene IL-24 or the viro-agent ONYX-01519, 20. Using these methods, potent antitumor effects and reduced side effects have been obtained with the combination of two regimens and a reduced dose. However, further improvements are still required to facilitate such strategies for clinical cancer therapy.
Previous studies in our laboratory have shown that ZD55-IL-24 could selectively replicate in the tumor cell lines Bcap37, BEL7404, HeLa, SMMC7721, HCT116, and SW620 and that overexpression of IL-24 suppressed tumor cell growth and induced apoptosis12. In this study, we used the chemo-gene-virotherapy strategy and comprehensively combined cisplatin with both IL-24 and ZD55. The combination of ZD55-IL-24 and cisplatin was first performed to treat a variety of tumor cells, and an empiric inhibition of tumor efficacy has been demonstrated. Western blot analysis has distinctly addressed the specific viral replication in BEL7404 and HeLa cells (Figure 1D). Notably, although ONYX-015 has been used extensively in cancer clinical trials, it has not been proven to be very efficient25, 26. Nevertheless, ZD55-IL-24, which is an oncolytic adenovirus that harbors the therapeutic gene IL-24, cannot specifically replicate and efficiently express the IL-24 gene in cancer cells (Figure 1). However, it does lead to robust cancer-specific cytotoxicity (Figures 2, 3). Thus, this combinatorial strategy with ZD55-IL-24 and cisplatin has become an intriguing prospect for clinical cancer therapy.
The purpose of this study was to identify the enhanced cytotoxicity of cisplatin with gene-virotherapy agent ZD55-IL-24 in cancer cells and to evaluate the negative effects in normal cells. The MTT analysis showed that conjugation of cisplatin with ZD55-IL-24 was obviously superior to cisplatin, ZD55-EGFP, ZD55-IL-24 alone, or ZD55-EGFP plus cisplatin (Figure 2). However, a difference in cytotoxicity among the six tumor cells exists after various treatments. The difference may be caused by the sensitivity of different tumor cells to the adenovirus, the IL-24 gene, the chemotherapy drug, or their different cellular mechanisms. Some reports have indicated that the adenovirus-mediated IL-24 gene can be significantly expressed in tumor cells and lead to the specific apoptosis of tumor cells6, 9, 12. IL-24 triggers the external pathway of cell apoptosis, such as the activation of the caspase family, including the cleavage of caspase 3, 9, and PARP. The intrinsic pathway of apoptosis involves mitochondria cytochrome c release. In addition, IL-24 can improve antitumor immunity and suppress tumor angiogenesis. It indicated that oncolytic adenovirus mediated IL-24 induced a strong cleavage of PARP by Western blot analysis (Figure 5). Hoechst33342 staining indicated that extensive chromatin condensation and nuclear fragmentation, which is a typical sign of cell apoptosis, was observed in the combined treatment of cisplatin with ZD55-IL-24 (Figure 6). However, the results have also shown that ZD55-EGFP exhibited a small antitumor effect and led to the apoptosis of tumor cells through the small cleavage of PARP compared with the ZD55-IL-24 plasmid (Figure 5). This suggests that the IL-24 gene has a significant role in tumor cell apoptosis. Oncolytic adenovirus can proliferate in tumor cells, lyse them, and achieve a vigorous tumor-killing effect10, 25, 26. Furthermore, some adenovirus structure proteins, such as E1A and E4, have an affect on the induction of tumor cell apoptosis.
ZD55-IL-24 had a dual role in both gene therapy and virotherapy, which led to a further improvement in the antitumor effect when it was combined with cisplatin. The mechanism of combined therapy includes an increased tumor killing effect, the promotion of tumor cell apoptosis, and reduced side effects in normal cells (Figures 2, 6, 7). The conjugation of ZD55-IL-24 and cisplatin may cause cell cycle arrest in tumors, stimulate an antitumor immune response, and exhibit a “bystander effect” to inhibit tumor angiogenesis and vascular epithelial growth factor. ZD55-IL-24 may also improve tumor cell sensitivity to cisplatin and reduce the resistance of cancer cells to cisplatin. The strong antitumor mechanism of this combination is still unclear and requires further investigation.
The severe side effects of high-dose chemotherapy with cisplatin are the essential obstacles in the treatment of solid tumors27, 28. With cisplatin and ZD55-IL-24 working together, the dose could be expected to not only dramatically enhance the tumor killing effect but also significantly reduce the toxicity in normal cells. The MTT assay in L-02 normal cells led us to determine the minimal dose (5 MOI ZD55-IL-24+3 μg/mL cisplatin) in the current test. The cytotoxicity assay, morphological evaluation, and apoptotic detection showed very slight damage to normal cells at the minimal dose (Figures 7, 8).
This study is the first to show the combined use of ZD55-IL-24 and cisplatin in cancer therapy. Conjugation of ZD55-IL-24 with cisplatin could cause remarkable cytotoxicity in several cancer cell lines and significantly abolish side effects in normal cells through a reduction in the required dose. In addition, we have demonstrated that the current chemo-gene-virotherapy (cisplatin+ZD55-IL-24) strategy is superior to the conventional chemo-gene (cisplatin+IL-24) or chemo-viro (cisplatin+ONYX-015) approach. We therefore conclude that the chemo-gene-virotherapy, with a combination of ZD55-IL-24 and cisplatin, may be a powerful and effective strategy for clinical cancer treatment.
Author contribution
Yi-gang WANG designed research; Yu-mei WU performed research; Xue-tian YUE, Yi YANG, Gong-chu LI and Na LI contributed new analytical tools and reagents; Kang-jian ZHANG and Yi-qiang WANG analyzed data; Yu-mei WU and Yi-gang WANG wrote the paper.
References
Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB . Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene 1995; 11: 2477–86.
Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB . The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene 2002; 21: 708–18.
Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, et al. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci USA 2001; 98: 10332–7.
Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, et al. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther 2003; 2: S23–S37.
Chada S, Mhashilkar AM, Liu Y, Nishikawa T, Bocangel D, Zheng M, et al. mda-7 gene transfer sensitizes breast carcinoma cells to chemotherapy, biologic therapies and radiotherapy: correlation with expression of bcl-2 family members. Cancer Gene Ther 2006; 13: 490–502.
Miyahara R, Banerjee S, Kawano K, Efferson C, Tsuda N, Miyahara Y, et al. Melanoma differentiation-associated gene-7 (mda-7)/interleukin (IL)-24 induces anticancer immunity in a syngeneic murine model. Cancer Gene Ther 2006; 13: 753–61.
Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE . Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther 2004; 9: 818–28
Chada S, Nemunaitis J, Tong A, Zhang Y, Su D, Mhashilkar A, et al. A Phase I dose-escalation study of Ad-mda7 (INGN 241) in patients with advanced carcinoma. Cancer Gene Ther 2001; 8 (Suppl 2): S3.
Chada S, Cunningham C, Zhang Y, Su D, Mhashilkar A, Ekmekcioglu S, et al. INGN 241 (Ad-mda-7) induces widespread apoptosis and activates the immune system in patients with advanced cancer. Mol Ther 2003; 7: S446.
Zhang ZL, Zou WG, Luo CX, Li BH, Wang JH, Sun LY, et al. An armed oncolytic adenovirus system, ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res 2003; 13: 481–99.
Liu XY, Qiu SB, Zou WG, Pei ZF, Gu JF, Luo CX, et al. Effective gene-virotherapy for complete eradication of tumor mediated by the combination of htrail (tnfsf10) and plasminogen k5. Mol Therapy 2005; 11: 531–41.
Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He L, et al. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Hum Gene Ther 2005; 16: 845–58.
Kelland L . The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007; 7: 573–84.
Rosenberg B, Vancamp L, Krigas T . Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965; 205: 698–9.
Morton RP, Rugman F, Dorman EB, Stoney PJ, Wilson JA, McCormick M, et al. Cisplatinum and bleomycin for advanced or recurrent squamous cell carcinoma of the head and neck: a randomized factorial phase III controlled trial. Cancer Chemother Pharmacol 1985; 15: 283–9.
Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, Loehrer PJ . Treatment of disseminated germ-cell tumors with cisplatin, bleomycin and either vinblastine or etoposide. N Engl J Med 1987; 316: 1435–40.
Pan QW, Liu BS, Liu J, Cai R, Wang YG, Qian C . Synergistic induction of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying TRAIL. Mol Cell Biochem 2007; 304: 315–23.
Pan QW, Zhong SY, Liu BS, Liu J, Cai R, Wang YG, et al. Enhanced sensitivity of hepatocellular carcinoma cells to chemotherapy with a Smac-armed oncolytic adenovirus. Acta Pharmacol Sin 2007; 28: 1996–2004.
Xiong J, Peng ZL, Tan X . Effects of adenoviral-mediated mda-7/IL-24 gene infection on the growth and drug-resistance of drug-resistant ovarian cancer cell lines. Sichuan Da Xue Xue Bao Yi Xue Ban 2007; 38: 433–6. Chinese.
Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of ONYX-015, a replication-selective adenovirus, in combination with cisplatin and 5-FU in patients with recurrent head and neck cancer. Nat Med 2000; 6: 879–85.
Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene 2001; 20: 7051–63.
Liu XY, Gu JF . Targeting gene-virotherapy of cancer. Cell Res 2006; 16: 25–30.
Hajra KM, Tan L, Liu JR . Defective apoptosis underlies chemoresistance in ovarian cancer. Adv Exp Med Biol 2008; 622: 197–208.
Uyama N, Hatano E, Maetani Y, Isoda H, Shibata T, Taura K, et al. Efficacy and toxicity of transcatheter arterial chemoembolization with cisplatin suspended in lipiodol for unresectable hepatocellular carcinoma. Gan To Kagaku Ryoho 2008; 35: 775–80.
Kirn D . Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther 2001; 8: 89–98.
Kirn D . Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): results of phase I and II trials. Expert Opin Biol Ther 2001; 1: 525–38.
Fillastre JP, Raguenez-Viotte G . Cisplatin nephrotoxicity. Toxicol Lett 1989; 46: 163–75.
Arany I, Safirstein RL . Cisplatin nephrotoxicity. Semin Nephrol 2003; 23: 460–4.
Acknowledgements
We thank Prof Xin-yuan LIU for his thorough review and approval of this article and Kan CHEN for her help with cell culture. This work was supported by the Zhejiang Science and Technology Support Plan (No 2007C33027), National Natural Science Foundation of China (No 30800093, No 30801379), Science and Technology Commission of Shanghai Municipality (No 06DZ22032), National Basic Research Program of China (973 Program, No 2004 CB51804), Key Project of the Chinese Academy of Science (No KSCX2-YW-R-09, R-04), and Zhejiang Sci-Tech University Grant 0616033 to Prof Xin-yuan LIU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Ym., Zhang, Kj., Yue, Xt. et al. Enhancement of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying MDA-7/IL-24. Acta Pharmacol Sin 30, 467–477 (2009). https://doi.org/10.1038/aps.2009.16
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2009.16
Keywords
This article is cited by
-
A novel bladder cancer - specific oncolytic adenovirus by CD46 and its effect combined with cisplatin against cancer cells of CAR negative expression
Virology Journal (2017)
-
Synergistic antitumor activity of triple-regulated oncolytic adenovirus with VSTM1 and daunorubicin in leukemic cells
Apoptosis (2016)
-
Combined therapy with CTL cells and oncolytic adenovirus expressing IL-15-induced enhanced antitumor activity
Tumor Biology (2015)
-
Synergistic suppression effect on tumor growth of hepatocellular carcinoma by combining oncolytic adenovirus carrying XAF1 with cisplatin
Journal of Cancer Research and Clinical Oncology (2015)
-
Combination of bladder cancer-specific oncolytic adenovirus gene therapy with cisplatin on bladder cancer in vitro
Tumor Biology (2014)