Abstract
Aim:
To investigate noncovalent interactions between borneol and human serum albumin (HSA) under near-physiological conditions.
Methods:
A 65-μm polydimethylsiloxane (PDMS) fiber was selected for sampling. The extraction temperature was kept at 37 °C, and the extraction time was optimized at 10 min. Borneol solutions of different concentrations were equilibrated in 600 μmol/L HSA and 67 mmol/L phosphate buffer solution (pH 7.4, 37 °C) for 24 h prior to solid phase microextraction (SPME) using headspace mode. The binding properties were obtained based on the calculation of extracted borneol amount using gas chromatography (GC) determination.
Results:
The headspace SPME extraction method avoided disturbance from the HSA binding matrix. The recovery showed good linearity for the borneol concentrations over the range of 0.4–16.3 μmol/L with a regression coefficient (R2) of 0.9998. The limit of detection and lower limit of quantitation were determined to be 0.01 μmol/L and 0.4 μmol/L, respectively. The binding constant and the percentage binding rate were estimated to be 2.4×103(mol/L)-1 and 59.5%, respectively.
Conclusion:
Headspace SPME coupled to GC is a simple, sensitive and rapid method for the study of borneol binding to HSA. The method may be applied in the determination of other protein binding properties in human plasma.
Similar content being viewed by others
Introduction
Drug action in living organisms is a result of a large number of pharmacological processes. As the most abundant protein in human plasma, human serum albumin (HSA) interacts with a wide range of endogenous and exogenous compounds. HSA binding affects the unbound (free) drug concentrations in blood; only the unbound drug compound is pharmacologically active and capable of crossing cell membranes, which significantly affects its distribution, metabolism, elimination, toxicity and biological activity. Because of its clinical and pharmaceutical importance, a great deal of attention has been paid to the study of HSA interactions with a number of natural or synthetic active compounds.
Numerous methods have been applied to study the binding properties of drugs to HSA, including equilibrium dialysis, ultrafiltration and gel filtration1, 2, 3. These techniques are usually time-consuming, can suffer loss of the analyte to membranes, and can create a shift in the binding equilibrium during separation. Solid phase microextraction (SPME) was initially developed as a simple extraction method. The extraction medium is the polymer coating of an optical silica fiber. The fiber can release the extracted analyte using heat with gas chromatography (GC), or an organic solvent with high performance liquid chromatography (HPLC). The main advantage of SPME is its elegant combination of the sampling, concentration and separation processes in a single step, hence reducing time, labor and polluting organic solvents. SPME is simple and low-cost and has been applied to various areas, including environmental, biological and pharmaceutical analysis4, 5, 6, 7, 8.
An interesting application is that SPME can be used in negligible-depletion extraction mode (nd-SPME) for free analyte determination in matrix samples9, 10, 11. Nd-SPME can be applied by exposing the fiber in the headspace above the sample (headspace nd-SPME) or direct immersion in the sample. The advantage of headspace nd-SPME sampling is that the matrix in the sample cannot interfere with the fiber. The equilibrium between the free analyte and the protein-bound analyte is not disturbed. Thus, the extracted amount of analyte is proportional to the free drug concentration without interference from the protein binding matrix.
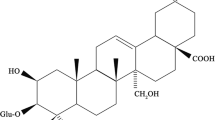
Borneol (Figure 1), a monoterpenoid alcohol, is a semi-volatile active drug component that is consumed widely, particularly in combined formulas for preventing and curing cardiovascular disease in traditional Chinese medicines12, 13, 14. Borneol has multiple biological and pharmacological activities, including inhibition of acetylcholine-mediated effects15, enhancing the adsorption of tetramethylpyrazine phosphate16, modulation of bone metabolism17 and GABA action18. Borneol was also approved by the US FDA as a flavor (21 CFR 172.515). To understand the pharmacokinetic and pharmacodynamic properties of borneol, it is necessary to study its binding properties to HSA. To our knowledge, this is the first report to study borneol-HSA binding properties.
In this paper, we simulated physiological conditions with a fixed HSA concentration (600 μmol/L), 67 mmol/L phosphate solution (pH 7.4, 37 °C) and serially increasing concentration of borneol solutions, which were pre-equilibrated prior to headspace nd-SPME extraction. The free amount of borneol in the binding solution was calculated from the linear response by GC determination. The borneol-HSA binding constant and binding percentage were also obtained.
Experimental
Instrumentation
An Agilent 6890N GC coupled with an FID detector and Agilent Chemstation software (Version A.10.02) (Wilmington, USA) was used throughout the study. A DB-WAXETR capillary column (30 m × 0.32 mm i.d., 0.5 μm film thickness) and 10 mL headspace vials fitted with septa and crimp caps were obtained from Agilent Technologies (Wilmington, USA). The SPME manual holder and fibers coated with Carboxen/Divinylbenzene/Polydimethylsiloxane (CAR/DVB/PDMS, 50/30 μm thickness), PDMS/DVB (65 μm thickness) and CAR/PDMS (75 μm thickness) were purchased from Supelco (Bellefonte, USA). These fibers were conditioned in the GC injection port at 250 °C prior to use. A Sartorius PB-10 pH meter (Sartorius, Germany) was employed to adjust the pH of the phosphate buffer solution. Water was purified with a Sartorius Arium 631 purification system (Goettingen, Germany).
Nitrogen gas (99.9999%) served as the carrier gas at a constant pressure of 8.0 psi. The column temperature was kept at 40 °C for 5 min, raised to 160 °C at 20 °C/min and held for 5 min, and then further to 220 °C at 30 °C/min and held for 2 min. The injection port and the detector were set at 240 °C and 250 °C, respectively. The fiber was inserted into the injection port for 1.5 min and then purged-off for 15 min to make sure there was no carry-over observed between samples. The inlet was switched to purge-on until the end of the run time, and the SPME fiber was removed from the injector simultaneously.
Standards and samples
Borneol (>99%) was obtained from National Institute for the Control of Pharmaceuticals and Biological Products (Beijing, China). HSA stock solution (200 g/L, Lot No 200602010) was obtained from Shanghai Institute of Biological Products (Shanghai, China). Analytical-grade 1,2-propylene glycol was obtained from Sinopharm Chemical Reagent Co (Shanghai, China).
Phosphate buffer solution (67 mmol/L, PBS) was prepared by dissolving the appropriate amount of sodium dihydrogen phosphate dehydrate in water and adjusting the pH at 7.4 with 1 mol/L NaOH. Borneol stock solution (10 mmol/L) was prepared by dissolving an appropriate amount of borneol in 1,2-propylene glycol. Borneol standard calibration solutions were prepared from the stock solution by dilution with 67 mmol/L PBS to a concentration range of 0.4 μmol/L to 16.3 μmol/L. Serial concentration of binding solutions of borneol-HSA (borneol: 1.0–20.4 μmol/L, HSA: 600 μmol/L) were prepared by mixing borneol stock solution and HSA stock solution appropriately in 10-mL headspace vials and kept at equilibrium at 37 °C for 24 h. The contents of 1,2-propylene glycol in the standard calibration solutions and binding solutions were all maintained at 0.2% (v/v) to ensure the sufficient solubility of borneol without obvious disturbance from possible competing binding to HSA.
Results and discussion
To investigate physiologically relevant binding properties, the pH value, the concentration of the PBS solution and the equilibrium temperature were set close to physiological conditions. The concentration of borneol in the binding solutions was measured around a possible therapeutic level.
A 65-μm PDMS/DVB fiber, 50/30-μm CAR/DVB/PDMS fiber and 75-μm CAR/PDMS fiber were evaluated based on their extraction performance. The 65-μm PDMS/DVB fiber showed a higher extraction capacity for borneol while the 50/30-μm CAR/DVB/PDMS and 75-μm CAR/PDMS showed some disturbance shift, perhaps caused by a minor affinity to other volatile impurities in the solutions. Thus, the 65-μm PDMS/DVB fiber was chosen for further optimization. During the extraction process, the extraction temperature was maintained at 37 °C to leave the existing equilibrium intact. The relationships of borneol freely dissolved in aqueous phase (Df), bound to HSA (Db), distributed in the headspace (Dh) and extracted from the coating fiber (Ds) are shown in Figure 2.
It is generally accepted11 that three requirements should be met for the correct application of negligible depletion SPME in matrix-binding studies: (1) there is equilibrium between the bound and the free fraction of the analyte; (2) the binding matrix does not interfere with the extraction; and (3) the depletion of the free analyte fraction is negligible. The equilibrium between borneol and HSA was investigated between 0.5 h and 48 h. It was found that the equilibrium could be reached at 24 h. To verify that the HSA binding matrix does not interfere with the analysis, SPME was applied to a sample vial containing only HSA at the selected concentration. No interference or new peak was observed in the GC chromatogram. For the last requirement, which is the most critical, there has been a debate. As the depletion can never be 0%, the limit has to be set until which the depletion is considered to be negligible. Some researchers have set the maximum limit of depletion to be 10%20, 21. The depletion was calculated by dividing the mass found to be adsorbed in the fiber by the total initial analyte mass in the solution. Based on the further considerations of precision and negligible depletion, the extraction time ranged from 5 to 45 min was investigated for the borneol solutions in the absence of HSA. It was confirmed that 10 min of extraction time could satisfactorily fulfill the both considerations. The amount of borneol partitioned in the headspace relative to the initial total amount was less than 1%, which was calculated by comparing the peak area counts in the headspace using SPME injection and using direct injection of the borneol standard calibration solutions. The relative standard deviation (RSD %) for the assay precision were determined to be 4.83% and 1.65% at low (0.4 μmol/L) and high concentrations (0.4 μmol/L) of borneol, respectively.
Representative chromatograms with HSA absent and present are shown in Figure 3. A significant decrease of area response for borneol was observed in the presence of HSA, indicating some binding behavior. The binding between borneol (D) and HSA (P) can be described by the following equilibrium19:

where Kp is the affinity constant of borneol-HSA binding complex, which is defined as:

where Pt was estimated as the initial concentration of the HSA in the binding solutions (Pt=600 μmol/L), and Df and Db were the concentration of freely dissolved and bound borneol in the binding solutions, respectively. Since Pt was much larger than Db in this study, equation (2) can be reduced to:

A series of borneol calibration solutions was prepared to construct a linear calibration for determining the free borneol concentration in the binding solutions. The calibration equation was constructed as the response area (y) versus the borneol concentration (x). We determined this to be y=93.01x+1.77 with a regression coefficient (R2) of 0.9998, over the borneol concentration range of 0.4−16.3 μmol/L. The limit of detection was determined to be 0.01 μmol/L with a signal-to-noise ratio of 3. The lower limit of quantitation (LLOQ), defined as the lowest concentration with standard deviation of accuracy within 10%, was 0.4 μmol/L. The freely dissolved borneol concentration (Df) in the presence of HSA was calculated from the above calibration equation by measuring the concentration of borneol in the headspace. The bound concentration (Db) could then be obtained through the mass balance in the binding solution:

where D0 is the total initial concentration of borneol in the binding solution. The unbound borneol fraction, fu, is defined as the ratio of Df to D0 at equilibrium:

The bound borneol fraction, fb, is defined as fb=1–fu, which is commonly referred to as the percentage of protein binding. Figure 4 shows that Df and D0 had a linear relationship (Df= 0.405D0, R2=0.9991), which meant that the percentage of protein binding was fb %=(1−0.405)×100=59.5%. The mean value of Kp was thus obtained from Equation (3) as 2.4×103(mol/L)−1. The value range of Kp suggested that the binding of borneol with HSA was moderate under near physiological conditions.
To fully understand the binding characteristics of borneol in human plasma, further studies of binding to other proteins in plasma, such as α-acid glycoprotein (AAG), lipoproteins, or others, could be conducted based on our proposed method of headspace nd-SPME extraction coupled with GC.
Conclusions
As borneol is one of many widely used active ingredients in traditional Chinese medicine, it is helpful to understand its pharmacokinetic and pharmacodynamic properties by studying its binding affinity to plasma proteins. This work demonstrates a convenient application of nd-SPME coupled with GC to study the binding of borneol to HSA. This method overcomes the drawbacks of traditional methods such as equilibrium dialysis or ultrafiltration, which suffer from the loss of volatile components in the binding or transfer process. The method can also prevent matrix effects or disturbance of uptake kinetics. Future studies will reveal borneol binding characteristics to other proteins in human plasma, such as AAG and lipoproteins. This method allows further protein-binding studies for other volatile or semi-volatile active ingredients in traditional Chinese medicines.
Author contribution
Dong-ying CHEN and Liang HU designed the research. Liang HU performed the research and analyzed the data. Dong-ying CHEN and Liang HU wrote the paper.
References
Frostell-Karlsson A, Remaeus A, Roos H, Andersson K, Borg P, Hamalainen M, et al. Biosensor analysis of the interaction between immobilized human serum albumin and drug compounds for prediction of human serum albumin binding levels. J Med Chem 2000; 43: 1986–92.
Kim HS, Hage DS . Chromatographic analysis of carbamazepine binding to human serum albumin. J Chromatogr B 2005; 816: 57–66.
Wang HL, Zou HF, Zhang YK . Quantitative study of competitive binding of drugs to protein by microdialysis/high-performance liquid chromatography. Anal Chem 1998; 70: 373–7.
Pawliszyn J . Solid phase microextraction: theory and practice. New York: Wiley; 1997.
Lord H, Pawliszyn J . Microextraction of drugs. J Chromatogr A 2000; 902: 17–63.
Lord H, Pawliszyn J . Evolution of solid-phase microextration technology. J Chromatogr A 2000; 885: 153–93.
Snow NH . Solid-phase micro-extraction of drugs from biological matrices. J Chromatogr A 2000; 885: 445–55.
Theodoridis G . Application of solid-phase microextraction in the investigation of protein binding of pharmaceuticals. J Chromatogr B 2006; 830: 238–44.
Vaes WHJ, Ramos EU, Verhaar HJM, Seinen W, Hermens JLM . Measurement of the free concentration using solid-phase microextraction: binding to protein. Anal Chem 1996; 68: 4463–7.
Kopinke FD, Pörschmann J, Remmler M . Sorption behavior of anthropogenic humic matter. Naturwissenschaften 1995; 82: 28–30.
Heringa MB, Hermens JLM . Measurement of free concentrations using negligible depletion-solid phase microextraction (nd-SPME). Trends Anal Chem 2003; 22: 575–87.
Xu RS . Natural products chemistry. Beijing: Science Press; 2004. p181.
Huang WD, Lv WQ . Research progress of borneol. China Pharm 2008; 17: 64–6.
Han GZ . Pharmacokinetics of Chinese traditional and herbal drugs. Beijing: China Medicinal Science & Technology Press; 1999. p 369–71.
Park TJ, Park YS, Lee TG, Ha HJ, Kim KT . Inhibition of acetylcholine-mediated effects by borneol. Biochem Pharm 2003; 65: 8390.
Xiao YY, Ping QN, Chen ZP . The enhancing effect of synthetical borneol on the absorption of tetramethylpyrazine phosphate in mouse. Int J Pharm 2007; 337: 74–9.
Mühlbauer RC, Lozano A, Palacio S, Reinli A, Felix R . Common herbs, essential oils, and monoterpenes potently modulate bone metabolism. Bone 2003; 32: 372–80.
Granger RE, Campbell EL, Johnston GAR . (+)- And (-)-borneol: efficacious positive modulators of GABA action at human recombinant α1β2γ2L GABAA receptors. Biochem Pharm 2005; 69: 1101–11.
Zambonin CG, Aresta A . SPME-LC with UV detection to study delorazepam-serum albumin interactions. J Pharm Biomed Anal 2002; 29: 895–900.
Poerschmann J, Zhang Z, Kopinke FD, Pawliszyn J . Solid phase microextraction for determining the distribution of chemicals in aqueous matrices. Anal Chem 1997; 69: 597–600.
Parkerton TF, Stone MA, Letinski DJ . Assessing the aquatic toxicity of complex hydrocarbon mixtures using solid phase microextraction. Toxicol Lett 2000; 273: 112–3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, L., Chen, Dy. Application of headspace solid phase microextraction for study of noncovalent interaction of borneol with human serum albumin. Acta Pharmacol Sin 30, 1573–1576 (2009). https://doi.org/10.1038/aps.2009.148
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2009.148
Keywords
This article is cited by
-
Study on the interaction between active components from traditional Chinese medicine and plasma proteins
Chemistry Central Journal (2018)