Abstract
Aim:
To explore the binding mode of 2-substituted 1-indanone derivatives with acetylcholinesterase (AChE) and provide hints for the future design of new derivatives with higher potency and specificity.
Methods:
The GOLD-docking conformations of the compounds in the active site of the enzyme were used in subsequent studies. The highly reliable and predictive three-dimensional quantitative structure-activity relationship (3D-QSAR) models were achieved by comparative molecular field analysis (CoMFA) and comparative molecular similarity analysis (CoMSIA) methods. The predictive capabilities of the models were validated by an external test set. Moreover, the stabilities of the 3D-QSAR models were verified by the leave-4-out cross-validation method.
Results:
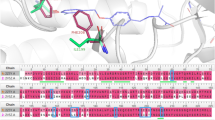
The CoMFA and CoMSIA models were constructed successfully with a good cross-validated coefficient (q2) and a non-cross-validated coefficient (r2). The q2 and r2 obtained from the leave-1-out cross validation method were 0.784 and 0.974 in the CoMFA model and 0.736 and 0.947 in the CoMSIA model, respectively. The coefficient isocontour maps obtained from these models were compatible with the geometrical and physicochemical properties of AChE.
Conclusion:
The contour map demonstrated that the binding affinity could be enhanced when the small protonated nitrogen moiety was replaced by a more hydrophobic and bulky group with a highly partial positive charge. The present study provides a better understanding of the interaction between the inhibitors and AChE, which is helpful for the discovery of new compounds with more potency and selective activity.
Similar content being viewed by others
Article PDF
References
Lahiri DK, Farlow MR, Greig NH, Sambamurti K . Current drug targets for Alzheimer's disease treatment. Drug Dev Res 2002; 56: 267–81.
Silman I, Sussman JL . Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacology. Curr Opin Pharmacol 2005; 5: 293–302.
Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL . Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Biol 1997; 4: 57–63.
Kryger G, Silman I, Sussman JL . Structure of acetylcholinest-erase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Struct 1999; 7: 297–307.
Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, et al. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci USA 1993; 90: 9031–5.
Harel M, Quinn DM, Nair HK, Silman I, Sussman JL . The X-ray structure of a transition state analog complex reveals the molecular origins of the catalytic power and substrate specificity of acetylcholinesterase. J Am Chem Soc 1996; 118: 2340–6.
Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL . Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3 Å resolution. FEBS Lett 1999; 463: 321–6.
Ravelli RB, Raves ML, Ren Z, Bourgeois D, Roth M, Kroon J, et al. Static Laue diffraction studies on acetylcholinesterase. Acta Crystallogr D Biol Crystallogr 1998; 54: 1359–66.
Inestrosa NC, Alvarez A, Perez CA, Moreno RD, Vicente M, Linker C, et al. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer's fibrils: possible role of the peripheral site of the enzyme. Neuron 1996; 16: 881–91.
Bartolini M, Bertucci C, Cavrini V, Andrisano V . Beta-amyloid aggregation induced by human acetylcholinesterase: inhibition studies. Biochem Pharmacol 2003; 65: 407–16.
Munoz-Muriedas J, Lopez JM, Orozco M, Luque FJ . Molecular modelling approaches to the design of acetylcholinesterase inhibitors: new challenges for the treatment of Alzheimer's disease. Curr Pharm Des 2004; 10: 3131–40.
Du DM, Carlier PR . Development of bivalent acetylcholinest-erase inhibitors as potential therapeutic drugs for Alzheimer's disease. Curr Pharm Des 2004; 10: 3141–56.
Soreq H, Seidman S . Acetylcholinesterase-new roles for an old actor. Nat Rev Neurosci 2001; 2: 294–302.
Sheng R, Lin X, Li J, Jiang Y, Shang Z, Hu Y . Design, synthesis, and evaluation of 2-phenoxy-indan-1-one derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem Lett 2005; 15: 3834–7.
Sheng R, Lin X, Li J, Jiang Y, Hu Y . Design, synthesis, and evaluation of 2-benzylidene-indan-1-one and 2-benzyl-indan-1-one derivatives as acetylcholinesterase inhibitors. In: Lee HZ, Hu YZ, editors. Proceedings of the 7th China-Japan Joint Symposium on Drug Design and Development; 22-26 Sep 2005, Hangzhou, China. Asian Federation of Medicinal Chemistry. Hangzhou: China; 2005. p31–p33.
Akula N, Lecanu L, Greeson J, Papadopoulos V . 3D QSAR studies of AChE inhibitors based on molecular docking scores and CoMFA. Bioorg Med Chem Letter 2006; 16: 6277–80.
SYBYL (Computer program). Version 7.0. St Louis, MO: Tripos Associates; 2004.
Clark M, Cramer RDI, van Opdenbosch N . Validation of the general purpose Tripos 5.2 force field. J Comput Chem 1989; 10: 982–1012.
Gasteiger J, Marsili M . Iterative partial equalization of orbital electronegativity: a rapid access to atomic charges. Tetrahedron 1980; 36: 3219–28.
ACD/Labs Online (I-Lab), pKa caculation. [cited 2006 Aug 6]. Available from URL: http://ilab.acdlabs.com/.
Gilson MK, Straatsma TP, McCammon JA, Ripoll DR, Faerman CH, Axelsen PH, et al. Open “back door” in a molecular dynamics simulation of acetylcholinesterase. Science 1994; 263: 1276–8.
Wlodek ST, Antosiewicz J, McCammon JA . Prediction of titration properties of structures of a protein derived from molecular dynamics trajectories. Protein Sci 1997; 6: 373–82.
Weiner SJ, Kollman PA, Case DA, Singh C, Ghio G, Alagona S, et al. A new force field for molecular mechanical simulation of nucleic acids and proteins. J Am Chem Soc 1984; 106: 765–84.
Jones G, Willett P, Glen RC, Leach AR, Taylor R . Development and validation of a genetic algorithm for flexible docking. J Mol Biol 1997; 267: 727–48.
Eldridge MD, Murray CW, Auton TR, Paolini GV, Mee RP . Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J Comput Aided Mol Des 1997; 11: 425–45.
Guo J, Hurley MM, Wright JB, Lushington GH . A docking score function for estimating ligand-protein interactions: application to acetylcholinesterase inhibition. J Med Chem 2004; 47: 5492–500.
Cramer RD III, Patterson DE, Bunce JD . Comparative molecular field analysis. 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 1988; 110: 5959–67.
Klebe G, Abraham U, Mietzner T . Molecular similarity indices in a comparative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity. J Med Chem 1994; 37: 4130.
Xie Q, Tang Y, Li W, Wang XH, Qiu ZB . Investigation of the binding mode of (-)-meptazinol and bis-meptazinol derivatives on acetylcholinesterase using a molecular docking method. J Mol Model 2006; 12: 390–7.
Sugimoto H, Yamanishi Y, Iimura Y, Kawakami Y . Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr Med Chem 2000; 7: 303–39.
Kaur J, Zhang MQ . Molecular modelling and QSAR of reversible acetylcholines-terase inhibitors. Curr Med Chem 2000; 7: 273–94.
Golbraikh A, Tropsha A . Beware of q2. J Mol Graph Model 2002; 20: 269–76.
Shi LM, Fang H, Tong W, Wu J, Perkins R, Blair RM, et al. QSAR models using a large diverse set of estrogens. J Chem Inf Comput Sci 2001; 41: 186–95.
Zuo Z, Luo X, Zhu W, Shen J, Shen X, Jiang H, et al. Molecular docking and 3D-QSAR studies on the binding mechanism of statine-based peptidomimetics with beta-secretase. Bioorg Med Chem 2005; 13: 2121–31.
Schrödinger (Computer program). Version 2006. Portland, OR: Schrödinger, LLC; 2006.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Natural Science Foundation of China (No 20572023), the Shanghai Key Basic Research Project (No 05JC14092), the Shanghai Pujiang Program (No 05PJ14034), and the Foundation of East China University of Science and Technology for Research (No YC0142101).
Rights and permissions
About this article
Cite this article
Shen, Ll., Liu, Gx. & Tang, Y. Molecular docking and 3D-QSAR studies of 2-substituted 1-indanone derivatives as acetylcholinesterase inhibitors. Acta Pharmacol Sin 28, 2053–2063 (2007). https://doi.org/10.1111/j.1745-7254.2007.00664.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1745-7254.2007.00664.x
Keywords
This article is cited by
-
ABCpred: a webserver for the discovery of acetyl- and butyryl-cholinesterase inhibitors
Molecular Diversity (2022)
-
Benefits of statistical molecular design, covariance analysis, and reference models in QSAR: a case study on acetylcholinesterase
Journal of Computer-Aided Molecular Design (2015)
-
Computational design of novel flavonoid analogues as potential AChE inhibitors: analysis using group-based QSAR, molecular docking and molecular dynamics simulations
Structural Chemistry (2015)
-
Protective Effects of a Piperazine Derivative [N-{4-[4-(2-methoxy-phenyl)-piperazin-1-yl]-phenyl} Carbamic Acid Ethyl Ester] Against Aluminium-Induced Neurotoxicity: Insights From In Silico and In Vivo Studies
Neurotoxicity Research (2015)
-
Docking of the alkaloid geissospermine into acetylcholinesterase: a natural scaffold targeting the treatment of Alzheimer’s disease
Journal of Molecular Modeling (2011)