Abstract
Aim:
To investigate whether the pharmacodynamics and pharmacokinetics of rabeprazole are dependent on CYP2C19 genotype status in healthy Chinese Han subjects.
Methods:
The CYP2C19 genotype status of healthy Chinese Han volunteers was determined using the polymerase chain reaction-restriction fragment length polymorphism method. Twenty healthy subjects volunteered to participate in the study. There were seven homozygous extensive metabolizers (homEM), six heterozygous extensive metabolizers (hetEM), and seven poor metabolizers (PM). All subjects were Helicobactor pylori-negative, which was determined by serology and 13C-urea breath tests. Rabeprazole (20 mg) was taken orally once daily in the morning for 8 days, and intragastric pH values were monitored for 24 h by Digitrapper pH after day 1 (single dose) and day 8 (repeated dose). Meanwhile, blood samples were collected at various time-points for 24 h after administration. The serum concentrations of rabeprazole were measured using high-performance liquid chromatography.
Results:
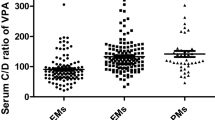
The mean area under the curve (AUC) values for rabeprazole differed among the three different genotype groups, with a relative ratio of 1.0, 1.3, and 1.8 after a single dose and 1.0, 1.1, and 1.7 after repeated doses in the homEM, hetEM, and PM groups, respectively. Mean AUC values for rabeprazole after a single dose and after repeated doses were significantly different between the homEM and PM groups, but not between the homEM and hetEM or hetEM and PM groups. No significant differences in intragastric pH median, pH>4 total time, and pH>4 time percentage of 24 h, were observed among the three different genotype groups after a single dose or after repeated doses of rabeprazole.
Conclusion:
In healthy Chinese Han subjects, the pharmacokinetics of rabeprazole are dependent on a certain degree on CYP2C19 genotype status; however, the acid-inhibitory efficacy of rabeprazole is not influenced significantly by CYP2C19 genetic polymorphism.
Similar content being viewed by others
Article PDF
References
Andersson T, Regardh CG, Dahl-Puustinen ML, Bertilsson L . Slow omeprazole metabolizers are also poor S-mephenytoin hydroxylators. Ther Drug Monit 1990; 12: 415–6.
Chiba K, Kobayashi K, Manabe K, Tani M, Kamataki T, Ishizaki T . Oxidative metabolism of omeprazole in human liver microsomes: cosegregation with S-mephenytoin 4′-hydroxylation. J Pharmacol Exp Ther 1993; 266: 52–9.
Adachi K, Katsube T, Kawamura A, Takashima T, Yuki M, Amano K, et al. CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole. Aliment Pharmacol Ther 2000; 14: 1259–66.
Sakai T, Aoyama N, Kita T, Sakaeda T, Nishiguchi K, Nishitora Y, et al. CYP2C19 genotype and pharmacokinetics of three proton pump inhibitors in healthy subjects. Pharm Res 2001; 18: 721–7.
Hokari K, Sugiyama T, Kato M, Saito M, Miyagishima T, Kudo M, et al. Efficacy of triple therapy with rabeprazole for Helicobacter pylori infection and CYP2C19 genetic polymorphism. Aliment Pharmacol Ther 2001; 15: 1479–84.
Miyoshi M, Mizuno M, Ishiki K, Nagahara Y, Maga T, Torigoe T, et al. A randomized open trial for comparison of proton pump inhibitors, omeprazole versus rabeprazole, in dual therapy for Helicobacter pylori infection in relation to CYP2C19 genetic polymorphism. J Gastroenterol Hepatol 2001; 16: 723–8.
Shu Y, Zhou HH . Individual and ethnic differences in CYP2C19 activity in Chinese populations. Acta Pharmacol Sin 2000; 21: 193–9.
de Morais SM, Goldstein JA, Xie HG, Huang SL, Lu YQ, Xia H, et al. Genetic analysis of the S-mephenytoin polymorphism in a Chinese population. Clin Pharmacol Ther 1995; 58: 404–11.
Yasuda S, Ohnishi A, Ogawa T, Tomono Y, Hasegawa J, Nakai H, et al. Pharmacokinetic properties of E3810, a new proton pump inhibitor, in healthy male volunteers. Int J Clin Pharmacol Ther 1994; 32: 466–73.
Ishizaki T, Chiba K, Manabe K, Koyama E, Hayashi M, Yasuda S, et al. Comparison of interaction potential of a new proton pump inhibitor, E3810, versus omeprazole with diazepam in extensive and poor metabolizers of S-mephenytoin 4′-hydroxylation. Clin Pharmacol Ther 1995; 58: 155–64.
Ishizaki T, Horai Y . Review article: cytochrome P450 and the metabolism of proton pump inhibitors–emphasis on rabeprazole. Aliment Pharmacol Ther 1999; 13 ( Suppl 3): 27–36.
Williams MP, Sercombe J, Hamilton MI, Pounder RE . A placebo-controlled trial to assess the effects of 8 days of dosing with rabeprazole versus omeprazole on 24-h intragastric acidity and plasma gastrin concentrations in young healthy male subjects. Aliment Pharmacol Ther 1998; 12: 1079–89.
Horai Y, Kimura M, Furuie H, Matsuguma K, Irie S, Koga Y, et al. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther 2001; 15: 793–803.
Inaba T, Mizuno M, Kawai K, Yokota K, Oguma K, Miyoshi M, et al. Randomized open trial for comparison of proton pump inhibitors in triple therapy for Helicobacter pylori infection in relation to CYP2C19 genotype. J Gastroenterol Hepatol 2002; 17: 748–53.
Ieiri I, Kishimoto Y, Okochi H, Momiyama K, Morita T, Kitano M, et al. Comparison of the kinetic disposition of and serum gastrin change by lansoprazole versus rabeprazole during an 8-day dosing scheme in relation to CYP2C19 polymorphism. Eur J Clin Pharmacol 2001; 57: 485–92.
Hussein Z, Granneman GR, Mukherjee D, Samara E, Hogan DL, Koss MA, et al. Age-related differences in the pharmacokinetics nd pharmacodynamics of lansoprazole. Br J Clin Pharmacol 1993; 36: 391–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Natural Science Foundations of the Ministry Education of Anhui Province of China (No 2003kj199).
Rights and permissions
About this article
Cite this article
Hu, Ym., Xu, Jm., Mei, Q. et al. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotype in healthy Chinese subjects. Acta Pharmacol Sin 26, 384–388 (2005). https://doi.org/10.1111/j.1745-7254.2005.00047.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1745-7254.2005.00047.x
Keywords
This article is cited by
-
Pharmacokinetics of CYP2C9, CYP2C19, and CYP2D6 substrates in healthy Chinese and European subjects
European Journal of Clinical Pharmacology (2018)
-
Individualized Therapy for Gastroesophageal Reflux Disease
Molecular Diagnosis & Therapy (2012)
-
Study of the pharmacokinetics and intragastric pH of rabeprazole given as successive intravenous infusion to healthy Chinese volunteers
European Journal of Clinical Pharmacology (2011)
-
Effect of CYP2C19 genotypes on the pharmacokinetic/pharmacodynamic relationship of rabeprazole after a single oral dose in healthy Chinese volunteers
European Journal of Clinical Pharmacology (2010)
-
Endoscopic Analysis of Gastric Ulcer after One Week's Treatment with Omeprazole and Rabeprazole in Relation to CYP2C19 Genotype
Digestive Diseases and Sciences (2008)