Abstract
Discotic liquid crystals (DLCs) are nanomaterials with sizes ranging from 2 to 6 nm, and they are emerging as one-dimensional organic semiconducting materials. Recently, hybridization of these materials with various metallic and semiconducting nanoparticles (NPs) to alter and improve their properties has been realized. This article provides an overview of the developments in the field of newly immersed discotic nanoscience, a sub-field of liquid crystal (LC) nanoscience. As this field is also of great interest to readers without an LCs background, a brief introduction of the LC field is given first, with special emphasis on DLCs. This is followed by various DLC-NP hybrid systems. Finally, an outlook into the future of this newly emerging, fascinating and exciting field of discotic nanoscience research is provided.
Similar content being viewed by others
Introduction
Over the past two decades, there has been an explosive development in the fields of nanostructured materials, including metallic nanoparticles (NPs) and nanorods (NRs), semiconducting quantum dots (QDs), carbon nanotubes (CNTs), fullerenes and graphene. The aim of experimental research has been to harvest the immense commercial technological potential of these materials, as well as gain an understanding of various fundamental processes at the nanometer scale.
Materials that have at least one dimension in the range of 1–100 nm are defined as nanomaterials. They possess unique properties, which differ from those of individual atoms or molecules and from those of bulk material. For example, the conductivity of metal NPs decreases significantly as their size decreases to one billionth of a meter; silver NPs, but not bulk silver, possess antibacterial activity; and the optical absorption and fluorescence properties of metal NPs are very different than in the bulk materials.1, 2 The science and technology of nano-sized materials, that is, nanoscience and nanotechnology, have received immense interest in nearly every field of science. The potential applications of nanomaterials in the fields of energy, computing, optics, catalysis, biosciences and medical sciences have been extensively discussed.3
Government support in many countries has enabled nanomaterials research to take center stage, allowing researchers from many different fields to pursue their work in the nano-realm. The field of nanoscience has quickly evolved during the past two decades, primarily because of our ability to see and touch nanoscale objects with the help of modern instruments, such as scanning tunneling microscope (STM), atomic force microscope (AFM), high-resolution transmission electron microscope (HRTEM) and field emission scanning electron microscope (FESEM). These developments have resulted in the addition of more than one million research studies on nanomaterials to the scientific literature over the last two decades (SciFinder search using the terms ‘nanoscience’ or ‘nanotechnology’ or ‘nanomaterials’ or ‘nanoparticles’).
On the other hand, liquid crystal (LC) science is a relatively mature, highly interdisciplinary science involving chemistry, physics, biology, engineering, medicine and now nanoscience and nanotechnology. Like nanomaterials, LCs also possess unique properties that differ from those of either crystals or liquids. From the emergence of this strong link between nanoscience and LCs, a new term, ‘Liquid Crystal Nanoscience,’ has evolved.4, 5 Assembling NPs into hierarchical and self-assembling structures is a fascinating way to prepare advanced functional materials.
The synergetic relationship of LCs possessing order and mobility at the molecular and supramolecular levels with the excellent electronic properties of NPs may produce advanced functional materials for next-generation electronic and opto-electronic applications.6, 7, 8 Efforts have been made to alter the physical properties of NPs and LCs either by attaching liquid crystalline molecules covalently to NPs or by adding a low concentration of NPs to a liquid crystalline matrix. A variety of zero-dimensional (0-D), one-dimensional (1-D) and two-dimensional (2-D) NPs have been explored to prepare LC-NP hybrid systems. After providing a very brief introduction to the LC field with a special emphasis on discotic liquid crystals (DLCs), this article focuses on the various aspects of DLC-NP hybrid systems with 0-D and 1-D NPs.
Liquid crystals
LCs are ubiquitous in everyday life in the form of LC display devices, which currently outnumber the people on earth. LCs has a crucial role in living systems and in biology.9 The combination of order and mobility, as exhibited by LCs, is the basic principle for self-organization and structure formation in living systems. Several biological molecules, such as lipids, carbohydrates, proteins and nucleic acids, have been found to exist in various liquid crystalline phases.9 The appearance of mesomorphism in large and nano DNAs has been related to the significant role of LCs in the evolution of biological information in the prebiotic world.10
Similar to biological self-assembly, LCs self-assemble into various structures with the help of many types of supramolecular interactions, such as van der Waals, dipolar and quadrupolar interactions; charge transfer; π–π interactions; metal coordination; and hydrogen bonding.11 The physical properties of this beautiful, delicate state of matter fall between those of the crystalline solid and amorphous liquid states; therefore, LCs are referred to as intermediate phases or mesophases. However, liquid crystalline states are true thermodynamically stable states of matter, just as solids, liquids and gases are. LCs possess the anisotropic (direction-dependent) properties of crystalline materials and the fluid properties of isotropic liquids. Molecular shape, nano-segregation of incompatible components, specific molecular interactions and self-organization are important factors that drive the formation of various LC phases in matter. These mesophases are often very sensitive to small external perturbations, such as electric and magnetic fields and surface effects, which are the basis for their information display applications.
The liquid-crystalline phases exist primarily in two types, thermotropic and lyotropic, depending on how the mesophases are obtained. Whereas lyotropic liquid-crystalline phases are obtained by dissolving amphiphilic compounds in suitable solvents over a range of concentrations and temperatures, thermotropic liquid-crystalline phases are achieved by the effect of temperature on pure compounds or a mixture of compounds either by heating the crystalline solid or by cooling an isotropic liquid. As the anisotropy of the molecular structure have a pivotal role in the formation of various mesophases, thermotropic LCs are primarily classified into three categories based on the shape of the mesogenic molecules: (a) rod-like (calamitic), (b) disc-like (discotic) and (c) bent-core or banana LCs. Providing a detailed description of these materials is beyond the scope of this study; however, additional information can be obtained from several books that are currently available on LCs. Here, only a very brief description of DLCs is provided.
Appropriately functionalized disc-shaped molecules (Figure 1) spontaneously self-assemble into nematic or columnar phases. This is a dynamic state of ordered molecular aggregation. The least-ordered, most-mobile mesophase is the nematic, ND, phase. In the nematic phase, molecules only possess orientational order with no long range positional order. As a result of the strong tendency of aromatic molecules to aggregate face-to-face, the majority of DLCs (approximately 95%) form columnar phases in which molecular discs stack one on top of the other to form a column, similar to a pile of coins. These columns of discs can then self-organize into different lattices, for example, hexagonal lattice (Colh), rectangular lattice (Colr) and oblique lattice (Colob). A few examples of DLCs are known to exhibit other phases, such as smectic and cubic phases.12
DLCs as 1-D organic semiconductors
In the columnar phase, molecules are packed very closely, similar to molecular packing in crystalline materials. The stacking gap (intra-columnar core-core separation) in a columnar mesophase is typically on the order of 0.35 nm, whereas the inter-columnar (between two adjacent columns) distance is typically 2–6 nm, depending on the length of lateral chains. Therefore, the interactions between neighboring molecules within the same column (intra-columnar) are much stronger than the interactions between neighboring columns (inter-columnar). Thus, the stacked molecular architecture, that is, columnar phase, can provide a facile path for the movement of generated charges via hopping from one molecule to another. Charge movement in these materials is expected to be quasi-1-D, as the conducting core of the discotic molecule is surrounded by insulating aliphatic chains (Figure 2).
The mobility of charge carriers is one of the most important factors in organic semiconductors for device applications. Organic single crystals with highly ordered structures possess very high charge carrier mobility, whereas amorphous organic materials with highly disordered molecular packing exhibit very low mobility. Thus, the charge carrier mobility may be directly correlated with the molecular ordering in organic materials. The columnar structures of DLCs fall between single crystalline and amorphous structures. However, LCs have the advantage of easy processability, particularly as thin films on an electrode surface, which is desirable in electronic devices.
Increasing the order in columnar structures is expected to enhance charge carrier mobility, and the molecular order may be enhanced by enlarging the size of polycyclic aromatic core as a result of intense π–π interactions. This concept has been well explored in the field of DLCs. An empirical relationship (∑μ1-D=3 exp(−83/n)[cm2 V−1 s−1]) between charge mobility (∑μ1-D) and core size has been proposed,13 although it did not hold for many large cores. A number of DLCs derived from large polycyclic aromatic hydrocarbon cores have been realized, and very high charge carrier mobility was observed, some examples of which are shown in Figure 3. This topic has been well covered in many reviews, such as references 14,15,16,17,18,19.
Like any other organic material, DLCs are basically insulators in their virgin state; however, they can be made electrically conducting by generating charges via chemical or photochemical doping. A number of dopants, such as bromine, iodine, AlCl3, NOBF4, trinitrofluorenone and ferrocenium ions, have previously been dispersed in DLCs to study the electrical conducting behavior of these materials.20, 21, 22, 23, 24 However, the most remarkable effect was observed in the case of trinitrofluorenone doping. When a triphenylene-based DLC, namely hexahexylthiotriphenylene (HHTT), was doped with a small amount (0.62 mol %) of trinitrofluorenone, the conductivity parallel to the columnar axis (σ||) increased by a factor of 107 or more.24 The direct current (DC) electrical conductivity of doped samples exhibits considerable anisotropy, σ||/σ⊥⩾1010.
Another route to improving the conducting properties of DLCs could be to incorporate metallic and semiconducting NPs into the supramolecular order of columnar phases. On the one hand, DLCs have been covalently attached to NPs. On the other hand, various functionalized NPs have been dispersed in DLCs. To date, most work has been performed on 0-D and 1-D NPs. These hybrid systems are discussed in the following sections.
Zero-dimensional NPs in DLCs
NPs with all dimensions at the nanoscale (<100 nm) are referred to as 0-D NPs. Some typical 0-D NPs incorporated into discotic liquid crystalline systems include gold NPs (GNPs), CdSe QDs and C60 fullerene. A brief description of these nanohybrid systems is provided below.
Gold NPs
Following the seminal work of Michael Faraday in 1857 on the reduction of gold salt by phosphorus,25 the scientific evaluation of GNPs received increased attention during the twentieth century. In recent times, a large number of methods have been developed to prepare GNPs with various shapes and sizes. Although a number of methods have been developed to prepare GNPs, including electrochemical, gas phase and liquid phase methods, the synthesis of GNPs in the liquid phase has received considerable attention because of its many advantages, such as large-scale synthesis, high yield and quality control. Under well-controlled reaction conditions, it is possible to produce monodisperse and uniform geometry NPs in high yield. GNPs are typically prepared via the reduction of gold salts in aqueous or organic media in the presence of surface stabilizers. These NPs are commonly referred to as monolayer-protected GNPs or gold clusters (particles smaller than 2 nm in size are often referred to as gold clusters).
The chemical reduction methods involve two steps: nucleation and successive growth of the particles. To produce monodisperse NPs, the nucleation step should be separated from the growth step. A variety of synthetic methods are now available to prepare monodisperse GNPs of almost any size.26, 27 Furthermore, the ligand-substitution reaction (Scheme 1) allows the incorporation of various surface functionalities onto NPs. Such monolayer-protected GNPs can be handled in a similar manner as general organic compounds because of their high stability under ambient conditions and their solubility in conventional organic solvents. Furthermore, a variety of chemical reactions can be performed on functionalized GNPs.
GNPs with mesogens
Three methods have primarily been used for the preparation of LC-GNP hybrid systems.27 All three varieties of LCs, that is, calamitic, discotic and banana LCs, have been used to prepare hybrids. In the simplest LC-GNP composites, monolayer-protected GNPs (often alkanethiol-protected GNPs prepared via the Brust–Schiffrin method28) were simply dispersed in various LCs. Solvent-mediated ligand substitution of such alkanethiol-protected GNPs with desired thiol-terminated mesogens provides mixed monolayer-protected GNPs having both alkane thiols and mesogens attached to the gold surface. To prepare GNPs passivated with mesogens only, the reduction of gold salt was performed in the presence of thiol-terminated mesogens. A flexible spacer is commonly used to attach mesogenic ligands to GNPs. Both mixed monolayer-protected GNPs and entirely mesogen-protected GNPs can be observed for mesogenity in the virgin state or may be further dispersed in liquid crystalline matrix to prepare composites.
Ascertaining the purity of the final isolated GNPs is of high importance. Purification of the monolayer-protected GNPs may be performed through repeated precipitation (dissolving the NPs in nonpolar solvents, such as dichloromethane or chloroform, then adding an excess of a polar solvent, such as methanol or ethanol, followed by centrifugation) or via chromatography or a combination of these methods. If the ligand is not removed completely, the system may act as monolayer-protected GNPs dispersed in the ligand. Moreover, if the ligand is mesogenic, it may give spurious results. Note that although the thiol-terminated ligand may not be mesogenic, its dimer (disulfide) formed in the reaction could be liquid crystalline. Therefore, it is very important to ensure, through the use of spectral and analytical techniques, that the GNPs are pure, particularly when reporting the mesogenic nature of virgin GNPs.
Rod-shaped mesogens or promesogens are the most commonly used ligands in the preparation of LC-GNP hybrid systems. GNPs covered with various calamitic molecules having a terminal thiol group connected linearly or laterally to the molecule, such as cyanobiphenyl thiol, cyclohexylphenoxy thiol and many derivatives of biphenyl thiol, have been reported to exhibit nematic, smectic and columnar phases.29, 30, 31, 32, 33, 34, 35, 36 Similarly, thiol-terminated bent-core ligands have also been used to prepare mesogen-passivated GNPs.37 A number of physical studies have been performed on these LC-GNP composites.38 As this study is primarily focused on DLCs, these above-mentioned calamitic and bent-core systems are not discussed in detail.
GNPs in discotic LCs
Compared with other liquid crystalline systems, very little work has been performed on DLC/GNP hybrid systems. We initiated this work by doping simple alkane thiol-passivated GNPs in DLCs.39 Hexanethiolate-stabilized gold clusters with a core diameter of 1.6 nm and an average composition of Au140[S(CH2)5CH3]53 were prepared and dispersed in three different DLCs, namely HHTT, hexapentyloxytriphenylene (HPT) and hexakis(4-nonylphenylethynyl)benzene, by mixing the two components in a sonicator, that is, DLC and GNP, in dichloromethane followed by removal of the solvent and drying under vacuum. The as-formed nanocomposites were analyzed using differential scanning calorimetry and polarizing optical microscopy (POM).
Pure HHTT exhibits a highly ordered helical phase at low temperatures in addition to a hexagonal columnar mesophase,40 whereas HPT displays only an ordered hexagonal columnar phase41 and hexakis(4-nonylphenylethynyl)benzene forms only a discotic nematic phase.42 The dispersion of GNPs in calamitic nematic LCs has been well studied by several researchers; however, the dispersion of GNPs in a discotic nematic LC could not be realized. The composite of discotic nematic LCs and GNPs shows macroscopic phase separation. However, note that a very large amount of GNPs (1:1), not favorable for dispersion, was attempted in a single experiment; therefore, it is very important to observe the dispersion of GNPs in small amounts and particularly in a room temperature discotic nematic LC. Alternatively, it is observed that the columnar phases of DLCs result in well-dispersed NPs in their matrices. Increasing the amount of GNPs in DLCs decreases the mesophase to isotropic temperature but the crystals to mesophase or mesophase to mesophase (helical phase to columnar phase) temperatures do not change significantly. Recent investigations performed by X-ray diffraction (XRD) studies on a GNPs-doped HHTT system described the formation of self-assembled superlattices of GNPs in the helical phase of HHTT.43 An increase in the electrical conductivity of the nanocomposite was observed because of the presence of GNPs in the matrix.
We subsequently designed and synthesized GNPs fully covered with triphenylene (TP) discotics to ascertain if such a functionalization could yield liquid-crystalline GNPs.44 Thus, the reduction of gold salt in the presence of thiol-functionalized triphenylene derivative yields discotic-decorated GNPs (Figure 4). In the virgin state, these discotic-decorated GNPs were found to be non-mesomorphic, but they could be easily dispersed into the columnar phase of a related triphenylene derivative, which is attributed to their chemical compatibility with one another. The XRD results indicate a random distribution of GNPs in the columnar matrix.
Remarkably, DC electrical conductivity measurements under ambient conditions show an enhancement of conductivity by six orders of magnitude (Figure 5) upon doping just 1% of GNPs into DLCs. Randomly distributed GNPs (Figure 6) can form charge transfer complexes when sandwiched between discotic cores, thus enhancing the conductivity of the system.44 Similar behavior has been observed when triphenylene-based discotics were doped with the electron-deficient trinitrofluorenone molecule.24 Holt et al.45 observed a similar enhancement (106) in electrical conductivity in the hexagonal columnar phase of a triphenylene-based DLC upon doping with 1% methylbenzene thiol-covered GNPs. The formation of small chain-like aggregates of GNPs upon applying a DC field was proposed. It is possible that GNPs are randomly dispersed in the bulk composite but begin to aggregate upon slow cooling from the isotropic phase (usually required to obtain homeotropic alignment of the columnar phase) or under an applied field. Nevertheless, a six orders of magnitude enhancement in conductivity under ambient conditions is highly significant from a device applications perspective. More studies on structure–property relationships are desirable to fully understand the mechanism of charge migration in these nanocomposites and to further explore their potential applications.
The variation in the measured DC conductivity values as a function of temperature for 1% triphenylene-gold nanoparticles (TP-GNPs) mixed with hexaheptyloxy-TP (H7TP) and neat H7TP. The conductivities shown were obtained while cooling from the isotropic phase. The vertical lines denote the phase transition temperatures obtained from the differential scanning calorimetry (DSC) studies.44
Although the triphenylene-functionalized NPs do not show columnar mesomorphism, they self-assemble into hexagonal patterns on surfaces, which is believed to arise from the strong π–π interactions between the triphenylene ligands of adjacent NPs.44 Shen et al.46 prepared triphenylene-protected GNPs with different spacer lengths and systematically investigated the self-assembled 1-D stripes and hexagonal close packed or disordered organization of GNPs as a function of the size of GNPs, alkyl chain lengths, interparticle π–π interaction and solvent hydrophilicity (Figure 7).
Schematic representation of hexagonal and stripe-like structure formation by triphenylene-covered gold nanoparticles (GNPs) depending on factors such as π–π interactions, space around TPs and solvent hydrophilicity (adapted from Shen et al.46).
We have also explored the dispersion of GNPs into the inverse-hexagonal columnar phase formed by an anionic surfactant, aerosol-OT, and normal lyotropic mesophase of a non-ionic surfactant, Triton X-100.47 The results indicate that GNPs are distributed outside the columns formed by both surfactants. In the Triton X-100 system, where a large free space is available in between the columns, the GNPs appear to prefer the outer surfaces, whereas in the aerosol-OT system, the outer surfaces of the columns are hydrophobic in nature, and therefore, the hydrophobic NPs prefer this region.
QDs in discotic LCs
The semiconductor inorganic NPs, commonly known as QDs, are the other 0-D NPs that have been extensively studied during the past decade because of their potential applications in the life sciences and materials science.48, 49, 50, 51 A number of QDs belonging to group II and VI elements (for example, CdSe, CdS, CdTe, ZnO, ZnSe and so on), group IV and VI elements (for example, PbS, PbSe, SnS and so on), and group III and V elements (for example, InP, InS, InN and so on) have been successfully prepared and studied for various properties. However, among all the QDs, CdSe QDs have received the most attention in materials science. A large number of synthetic methods have been developed52, 53, 54 after the pioneering work of Murray et al.55 on the synthesis of high-quality monodisperse Cd-based QDs. On the one hand, mesophases have been used to prepare these NPs.56 On the other hand, a number of QDs have been dispersed in various calamitic LCs with the hypothesis that such LC-QD nanocomposites may exhibit mutually beneficial effects on various electro-optical properties.57
To observe the dispersion of QDs in a DLC, we prepared organic-soluble CdSe QDs of two different sizes, 2.4 and 3.5 nm, and dispersed them in a columnar matrix of hexabutyloxytriphenylene (H4TP) DLC.58 The synthesized QDs were characterized by transmission electron microscopy (TEM), UV-Vis spectroscopy and photoluminescence spectroscopy. The TEM results confirm the synthesis of spherical particles with uniform sizes and shapes. The sharpness in the photoluminescence spectra of the QDs with a full width at half maximum of <30 nm indicates that the size distribution of the particles was very narrow. Both the absorption and emission spectra show an apparent redshift, as expected, with the increase in the size of the particle. Four composites of each size QD with H4TP DLC (1%, 2%, 3% and 5% of CdSe QDs in H4TP) were prepared by mixing the two components in chloroform. The solution was sonicated for a few minutes, followed by removal of the solvent and drying in vacuum.
The thermophysical properties, which were investigated using POM, differential scanning calorimetry and XRD, indicate that QDs disperse in H4TP without disturbing its mesomorphic nature. The results were quite similar to the dispersion of GNPs in DLCs. The XRD results indicate that the hexagonal columnar lattice of the LC is unaffected by the insertion of QDs in composites. Similar to GNPs, a random distribution of QDs (Figure 6) in the columnar phase was expected.
It is well known that QDs can be used as electron acceptors in organic–inorganic blends, and this property has been exploited in creating hybrid polymer–inorganic solar cells.59 To determine the effect of QDs on the conductivity of TP DLC, DC conductivity studies on QD-H4TP composites having 1% of 2.4 nm CdSe QDs were performed. In comparison with the conductivity of pure H4TP, the conductivity of the composite was found to be enhanced by two orders of magnitude. The enhancement in the conductivity could be a result of the formation of electron donor–acceptor interactions between the electron-rich organic triphenylene and the inorganic semiconductor.
Fullerenes in discotic LCs
Fullerenes, also called as buckyballs or buckminsterfullerenes, are hollow spheres composed of carbon atoms. This new allotrope of carbon was discovered in 1985, and since then, it has attracted considerable attention in the fields of supramolecular chemistry and materials science. Among the various fullerenes, such as C60, C70 and C76, the [60]fullerene (C60) has received the most attention in scientific fields. The C60 molecule is a sufficiently strong electron acceptor capable of accepting from one to six electrons to form the corresponding anions. Hence, the use of fullerene as an electron acceptor allows the lifetime of charge-separated states to be increased by several orders of magnitude compared with other known donor–acceptor systems. The donor–acceptor conjugates of C60 with various organic donors have recently been extensively studied as photoinduced electron and/or energy transfer systems, and highly efficient photovoltaic cells have been prepared from these conjugates.
As C60 is a molecular material, its synthesis, purification and characterization can be achieved efficiently with reproducibility. Moreover, it can be easily chemically functionalized to prepare various processable C60 derivatives. Recently, C60 and its derivatives have been dispersed in thermotropic LCs in an attempt to improve the physical properties of the host material. Both calamitic and discotic LCs have been used to study the effects of C60 doping on phase behavior.60 It is quite interesting to see how spherical C60 molecules organize in the mesophases and how their properties are affected in the supramolecular organization.
Although C60 does not show any discernible change in the transition temperature for nematic LCs, dendritic discotic LCs form supramolecular complexes with C60, thereby modifying the LC phase structure as well as substantially enhancing the isotropic phase transition temperature. Recently, several efforts have been made to covalently link various mesogens to C60. Here, only the covalent attachment of discotic mesogens is presented briefly.
There are two approaches that have pursued for covalently functionalizing C60 to yield liquid crystalline materials. The first is to functionalize C60 with discotic malonate derivatives via the use of the Bingel reaction. The second involves the Prato reaction via 1,3-dipolar cycloaddition reaction of C60 with an aldehyde terminated discotic molecule. Two representative examples are shown in Scheme 2.
Discotic mesogens, such as triphenylene,61, 62, 63 phthalocyanines64, 65, 66, 67, 68, 69, 70 and porphyrins,71, 72, 73 have been attached to fullerene units. Manickam et al.61 prepared a non-liquid crystalline bis-triphenylene C60 adduct 5 (Figure 8) via the Bingel reaction. Bushby et al.62 prepared two triphenylene-C60 adducts; in one, 6, the C60 is attached to one TP molecule, whereas in the other, 7, two identical TP moieties are connected to C60 via the Bingel reaction. They observed a 2-D hexagonal superlattice from the ordering of fullerenes within the hexagonal columnar liquid-crystal matrix formed by a fullerene derivative and a hexaphenyl hexaazatriphenylene discotic. It is believed that to maximize fullerene–fullerene contact, the fullerenes form chains that wrap around the central column in every group of seven columns. Yang et al.63 utilized the 1,3-dipolar cycloaddition reaction to prepare TP-C60 adduct 8. This compound was reported to be liquid crystalline.
Escosura et al.64 dispersed a phthalocyanine (Pc)-functionalized fullerene derivative 9 (Figure 9) in a columnar liquid crystalline phthalocyanine. The doping does not disturb the phase behavior of the host, as observed by optical microscopy and calorimetric studies; however, on the basis of X-ray diffraction studies of the blends, an alternating stacking of the host molecules and the dyads in the columns was proposed. Geerts et al.65 prepared liquid crystalline C60-Pc adducts 10 via linking tetralkoxy-Pc derivatives to the C60. It was observed that the length of the spacer between the Pc and the fullerene moieties significantly affects the thermotropic properties. Ince et al.66 also realized the importance of the spacer in inducing mesomorphism in C60-Pc diads. Compound 11 with a rigid spacer does not show liquid crystalline properties, whereas compound 12 with a flexible spacer self-organizes in a rectangular columnar mesophase. Hayashi et al.67 covalently linked a Pc donor with a fullerene acceptor. The columnar structure formed by compound 13 exhibits highly efficient charge transport properties.
During the synthesis of Pc-C60 dyad 2 via the Bingel reaction (Scheme 2), Ohta et al. observed the formation of 2:1 and 3:1 Pc-C60 side-products.68 However, they succeeded in the synthesis of pure Pc-C60 dyad 4 via the Prato reaction. These dyads show spontaneous homeotropic alignment in their columnar tetragonal mesophase, which is highly beneficial for device fabrication.
The dispersion of functionalized C60, such as pyridyl fullerene (PyC60) and 1-(3-methoxy-carbonyl)-propyl-1-1-phenyl-(6,6)C61 (PCBM), in phthalocyanine-based DLCs does not alter the columnar structure of the parent DLC.69, 70 These blends have been successfully used to fabricate solar cells that have power conversion efficiencies of up to 4.1%. Similarly, bilayer and bulk heterojunction solar cells have been prepared from liquid crystalline porphyrin donor and C60 acceptor layers.71 A few porphyrin DLCs have also been used to covalently attach to the C60 molecule. Wang et al.72, 73 reported the synthesis of trans-di-C60-substituted Zn porphyrin derivative 14 and porphyrin-C60 dyad 15 (Figure 10). These materials are reported to form supramolecular structures with an alternating arrangement of fullerenes and Zn porphyrins. Thus, it generates a donor–acceptor separated structure of C60 and Zn porphyrins in bulk, which is very interesting for the fabrication of solar cells.
1-D NPs in DLCs
NPs in which one dimension is out of the nanoscale while the other (diameter) is in the nano regime can be termed as 1-D nanomaterials. 1-D nanomaterials include nanotubes, NRs, nanowires and so on. These nano architectures have potential applications as nonlinear optical materials, photo-sensors, memory devices and so on. Similar to LCs, 1-D NPs are anisotropic materials; hence, their properties are direction dependent. Therefore, control over their orientation in the desired direction is crucial for many device applications. The dispersion of 1-D NPs into LCs is one of the attractive approaches by which the alignment of 1-D NPs could be accomplished. Although a variety of 1-D NPs, such as CNTs, GNRs, CdS NRs, ZnS NRs and Fe2O3 NRs, have been dispersed in calamitic LCs, only CNTs and GNRs have currently been doped into DLCs. A brief description is provided below.
CNTs in discotic LCs
CNTs are well-ordered, all-carbon, hollow cylinders of graphite with a high aspect ratio. As a result of their exciting electrical, mechanical and thermal properties, CNTs have emerged as one of the most widely studied nanomaterials during the past two decades. Three different types of CNTs, single-wall CNTs (SWNTs), double-wall CNTs and multi-wall CNTs (MWNTs), have been reported, but only SWNTs and MWNTs have been studied in detail. CNTs can be either metallic or semiconducting depending on the sheet direction about which the graphite sheet is rolled to form a nanotube cylinder. Both SWNTs and MWNTs have been dispersed in many LCs, and their effects on the electro-optical properties of LCs have been extensively studied by several researchers.4, 6, 74, 75
DLCs and CNTs have many similarities, for example, both are anisotropic materials; both possess self-assembling properties and often form hexagonal aggregates; and both exhibit 1-D conducting properties and have thus been considered as ‘molecular wires.’ These similarities prompted us to prepare DLC-CNT hybrid systems.76, 77 Initially, we attempted to disperse simple acid-purified SWNTs into the columnar matrix of H4TP in small quantities. However, we observed that the purified SWNTs form aggregates in the mesophase as observed under POM (Figure 11b). This could be a result of the noncompatibility of their chemical nature. With the hypothesis that the functionalization of SWNTs with discotic molecules may generate liquid crystalline SWNTs, or will at least have better dispersibility, we attempted the chemical functionalization of SWNTs with DLCs. Thus, classical esterification of acid-purified carboxylic acid-bearing SWNTs with a hydroxyl-functionalized triphenylene derivative yields functionalized nanotubes (f-SWNTs; Scheme 3). The formation of f-SWNTs was confirmed by spectral, microscopic and thermogravimetric analyses.
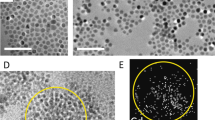
Although the as-prepared discotic-decorated SWNTs were found to be non-liquid crystalline in nature, they were soluble in common organic solvents, such as dichloromethane and chloroform, and therefore, they could be easily dispersed in organic-soluble DLCs. Thus, nanocomposites of f-SWNTs and H4TP were prepared, and their LC properties were investigated using POM, differential scanning calorimetry and XRD. The composites exhibited the classical texture of columnar phases on cooling from the isotropic liquid state, and no aggregations of CNTs were observed (Figure 11c). The insertion of CNTs does not significantly alter phase transition temperatures. The X-ray diffraction studies conducted on pure H4TP and 10 wt% f-SWNTs-doped H4TP revealed a significant shift in the first-order reflection toward larger d-spacings, indicating insertion of the SWNTs in the supramolecular ordering of the columnar phase occupying the space between the disc columns (Figure 12).
Later, we also studied the dispersion of simple octadecylamine-functionalized SWNTs in the columnar phases of triphenylene and rufigallol-based discotic monomers and polymers.77 These materials exhibit mesomorphism at room temperature. However, unlike discotic-functionalized SWNTs, which are more compatible with a discotic columnar phase, only a maximum of 2 wt% of SWNTs can be homogeneously dispersed in the columnar matrix. The π–π interactions of triphenylene molecules surrounding the SWNTs with the columnar phase forming triphenylene molecules likely stabilize the dispersion of f-SWNTs. Such room-temperature liquid-crystalline nanocomposites, with broad mesophase ranges and complimentary electronic properties, may be important for many device applications because the aligned CNTs can be reoriented in the desired direction using established LC alignment techniques.
Recently, Lee at al.78 reported that discotic ionic LCs derived from triphenylene core serve as excellent dispersants for pristine SWNTs. When sheared, the nanocomposites exhibit anisotropic conducting properties, and the shear-induced orientation of the SWNTs is maintained for a long period at room temperature. Lyotropic hexagonal columnar phases have also been successfully used to disperse SWNTs.79, 80 X-ray studies indicated that SWNTs are inserted in the columnar phase of lyotropic LCs. The nanocomposite can be transferred onto a solid substrate, thus obtaining aligned thin filaments of CNTs.
GNRs in columnar mesophases
Among the metal and semiconductor NRs, only gold NRs (GNRs) have currently been dispersed in DLCs. GNRs display two surface plasmon bands: a weak transverse band, similar to spherical GNPs, and a strong longitudinal band in the near-infrared region. The position of the longitudinal band red-shifts as the aspect ratio increases. This tunability of the plasmon band offers several applications for GNRs in bioscience.81, 82 A number of methods have been developed to prepare GNRs with different aspect ratios.81, 82, 83, 84 However, the seed-growth methods developed by Murphy83 and El-Sayed84 have gained more importance because of their simplicity and high yield of well-defined monodispersed GNRs.
To disperse GNRs in DLCs, we followed85 the method of Mostafa El-Sayed84 for the preparation of GNRs approximately 15 nm wide and 40 nm long with an aspect ratio of 2.7. The cetyltrimethylammonium bromide-coated GNRs were treated with dodecanethiol to obtain organic-soluble GNRs. A well-known triphenylene DLC, HPT, was used to disperse these GNRs. Nanocomposites of HPT and GNRs were prepared by mixing dichloromethane solutions of the two components in a sonicator, followed by removal of the solvent under vacuum. Next, several composites were prepared and characterized by UV-Vis spectroscopy, POM, differential scanning calorimetry, XRD and conductivity studies. The results indicate that similar to SWNTs (Figure 12), GNRs are inserted in between the columns of discotic molecules. HPT-GNR nanocomposites exhibit a marked increase in electrical conductivity compared with virgin systems.
GNRs embedded in discotic nanoribbons
The self-aggregation behavior of DLCs can be exploited to prepare nanofibers. The formation of 1-D nanostructures composed of discotic molecules with the π planes stacking in the direction of the long axis of the nanostructure has received considerable interest because of the potential of such systems for electronic devices. Xiao et al.86 reported that a discotic contorted hexabenzocoronene derivative organizes into molecular stacks and that these stacks organize into cables or fibers (Figure 13). Furthermore, a field effect transistor was constructed using an isolated nanofiber.
(a) Transmission electron microscope (TEM) images of fibers of 1 on a lacey carbon grid. (b) Higher magnification image of an individual fiber. (c) Electron diffraction pattern from a single hexabenzocoronene (HBC) fiber. (d) Rectangular arrangement of 1. (e) Packing of the columns into a fiber along the c-axis. Reproduced with permission from reference Xiao et al.86 (Copyright (2006) American Chemical Society).
Nanowires derived from triphenylene, perylene and decacyclene discotics have been used for the sensing of chemical explosives.87, 88, 89 The incorporation of GNRs in these discotic nanowires may provide better 1-D conducting properties. With this objective, we observed the formation of nanoribbons of undoped and GNR-doped triphenylene discotics via simple solution processing. When a solution of HPT or HPT-GNR composites in chloroform was added to methanol, discotic nanoribbons were formed. Scanning electron microscope (SEM) images (Figure 14) clearly demonstrate the formation of long ribbon-like structures for both HPT and GNR composites. These ribbons could be formed by the coming together of many hexagonal columns. In GNR-doped HPT nanoribbons, the GNRs are aligned with their long axis parallel to the length of the ribbon, as evident from the dark-field scanning transmission electron microscopy (STEM) image of 5% GNR-doped TP nanoribbons (Figure 14c). GNRs with a length of approximately 40 nm, identical to those observed in the TEM images, can be clearly observed along the length of the fibers, and no bright spots corresponding to the length or width of the GNRs are observed along the width of the fibers.
(a) Scanning electron microscope (SEM) images of randomly oriented fibers of hexapentyloxytriphenylene (HPT); (b) fibers formed by HPT-gold nanorod composites and (c) dark field STEM images of gold nanorods embedded in HPT nanoribbons. The bright spots in the fibers represent the gold nanorods embedded in the ribbon with their longitudinal axis parallel to the length of the ribbon.83
Thus, it may be concluded that p-stacking of discotic molecules creates columnar structures of discotics, primarily as a result of self-assembly. Furthermore, these columns grow into ribbon-like secondary structures upon proper solution processing. Similar processing of NP-doped DLC systems results in the formation of NPs embedded in discotic ribbons. These nanocomposites show enhanced conductivity because of the insertion of NPs in the ribbon-like structures; therefore, these composites may be better materials for devices such as field effect transistors.
Summary and outlook
The field of LC nanoscience is a very young and emerging field derived from two well-developed fields: LCs and nanoscience/nanotechnology. Discotic LC nanoscience, which is a subfield of LC nanoscience, has recently emerged in the scientific literature and has made significant advances during the past few years. A variety of DLC-NP hybrid systems have been prepared and studied because of their interesting properties. These hybrid systems can find applications in a wide range of fields, including photovoltaic solar cells, thin film transistors, photoconductors and so on. On the one hand, DLCs have been covalently attached to NPs; on the other hand, NPs such as CNTs, GNPs, GNRs and QDs have been dispersed in DLCs. Although NP-linked discotic molecules do not exhibit mesomorphism in the virgin state, it has been observed that various functionalized and even some unfunctionalized NPs can be well dispersed in the columnar phase of DLCs in small amounts without affecting their mesomorphic properties. The dispersion of NPs in DLCs imparts significant changes in their physical properties, for example, the electrical conductivity of the system increases by several orders of magnitude compared with the pure discotic system.
Limitless variations are possible, and several hundred DLCs have already been prepared and characterized. A variety of NPs with different shapes and sizes can be prepared to disperse in these DLCs. Unlike NP-calamitic LC hybrid systems, which have been extensively studied for many physical properties, such as dielectric properties, electro-optical properties, memory effects, photoluminescence and fluorescence confocal polarizing microscopy, to date, only a few physical studies, such as the electrical conductivity and dielectric behavior of discotic LC-NP composites, have been explored. Additional studies, such as charge mobility, alignment and anisotropic physical properties, have to be undertaken to realize their full potential for application in various devices. Discotic liquid crystalline NPs and DLC-NP composites may provide the next generation of advanced functional and/or engineered materials for many device applications that can only be realized as the field progresses.

Schematic representation of the synthesis of alkanethiol-protected gold nanoparticles (GNPs) and subsequent surface modification via ligand substitution reaction.

Covalent attachment of discotic moieties to C60 via Bingel and Prato reactions.

Schematic representation for the synthesis of discotic-decorated single-wall carbon nanotubes (SWNTs).
References
Daniel, M.-C. & Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 104, 293–346 (2004).
Burda, C., Chen, X., Narayanan, R. & El-Sayed, M. A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 105, 1025–1102 (2005).
Rao, C. N. R., Müller, A. & Cheetham, A. K. (eds) The Chemistry of Nanomaterials: Synthesis, Properties and Applications, Wiley-VCH, Weinheim, (2004).
Bisoyi, H. K. & Kumar, S. Liquid-crystal nanoscience: an emerging avenue of soft self-assembly. Chem. Soc. Rev. 40, 306–319 (2011).
Garbovskiy, Y. A. & Glushchenko, A. V. Liquid crystalline colloids of nanoparticles: preparation, properties, and applications. Solid State Phys. 62, 1–74 (2011).
Stamatoiu, O., Mirzaei, J., Feng, X. & Hegmann, T. Nanoparticles in liquid crystals and liquid crystalline nanoparticles. Top. Curr. Chem. 318, 331–393 (2012).
Nealon, G. L., Greget, R., Dominguez, C., Nagy, Z. T., Guillon, D., Gallani, J.-L. & Donnio, B. Liquid crystalline nanoparticles: hybrid design and mesophase structures. Beilstein J. Org. Chem. 8, 349–370 (2012).
Lagerwall, J. P. F. & Scalia, G. A new era of liquid crystal research: applications of liquid crystals in soft matter nano-, bio- and microtechnology. Current Appl. Phys. 12, 1387–1412 (2012).
Hamley, I. W. Liquid crystal phase formation by biopolymers. Soft Matter 6, 1863–1871 (2010).
Nakata, M., Zanchetta, G., Chapman, B. D., Jones, C. D., Cross, J. O., Pindak, R., Bellini, T. & Clark, N. A. End-to-end stacking and liquid crystal condensation of 6– to 20–base pair dna duplexes. Science 318, 1276–1279 (2007).
Kumar, S. Self-organization of disc-like molecules: chemical aspects. Chem. Soc. Rev. 35, 83–109 (2006).
Kumar, S. Chemistry of Discotic Liquid Crystals: From Monomers to Polymers, CRC Press: Boca Raton, FL, (2011).
van de Craats, A. M. & Warman, J. M. The core-size effect on the mobility of charge in discotic liquid crystalline materials. Adv. Mater. 13, 130–133 (2001).
Pisula, W., Feng, X. & Mullen, K. Charge-carrier transporting graphene-type molecules. Chem. Mater. 23, 554–567 (2011).
Sergeyev, S., Pisula, W. & Geerts, Y. H. Discotic liquid crystals: a new generation of organic semiconductors. Chem. Soc. Rev. 36, 1902–1929 (2007).
Wu, J., Pisula, W. & Mullen, K. Graphenes as potential material for electronics. Chem. Rev. 107, 718–747 (2007).
Kato, T., Mizoshita, N. & Kishimoto, K. Functional liquid-crystalline assemblies: self-organized soft materials. Angew. Chem. Int. Ed. 45, 38–68 (2006).
Ohta, K., Hatsusaka, K., Sugibayashi, M., Ariyoshi, M., Ban, K., Maeda, F., Naito, R., Nishizawa, K., van de Craats, A. M. & Warman, J. M. Discotic liquid crystalline semiconductors. Mol. Cryst. Liq. Cryst. 397, 25–45 (2003).
Hanna, J.-I. in: Liquid Crystalline Semiconductors (eds Bushby R. J., Kelly S. M., O’Neill M.) Ch. 2, 39–64 Springer: Dordrecht, (2013).
van Keulen, J., Warmerdam, T. W., Nolte, R. J. M. & Drenth, W. Electrical conductivity in hexaalkoxytriphenylenes. Recl. Trav. Chim. Pays-Bas 106, 534–536 (1987).
Vaughan, G. B. M., Heiney, P. A., McCauley, J. P. Jr. & Smith, A. B. III Conductivity and structure of a liquid-crystalline organic conductor. Phys. Rev. B 46, 2787–2791 (1992).
Boden, N., Bushby, R. J. & Clements, J. Mechanism of quasi one dimensional electronic conductivity in discotic liquid crystals. J. Chem. Phys. 98, 5920–5931 (1993).
Kumar, P. S., Kumar, S. & Lakshminarayanan, V. Electrical conductivity studies on discotic liquid crystal-ferrocenium donor-acceptor systems. J. Phys. Chem. B 112, 4865–4869 (2008).
Balagurusamy, V. S. K., Krishnaprasad, S., Chandrasekhar, S., Kumar, S., Manickam, M. & Yelamaggad, C. V. Quasi-one dimensional electrical conductivity and thermoelectric power studies on a discotic liquid crystal. Pramana 53, 3–11 (1999).
Faraday, M. The Bakerian lecture: experimental relations of gold (and other metals) to light, phil. Trans. R. Soc. Lond 147, 145–181 (1857).
Zhao, P., Li, N. & Astruc, D. State of the art in gold nanoparticle synthesis. Coordination Chem. Rev. 257, 638–665 (2013).
Louis, C. & Pluchery, O. (eds). Gold Nanoparticles for Physics, Chemistry and Biology, Imperial College Press: London, (2012).
Brust, M., Walker, M., Bethell, D., Schiffrin, D. J. & Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. Chem. Commun. 7, 801–802 (1994).
Kanayama, N., Tsutsumi, O., Kanazawa, A. & Ikeda, T. Distinct thermodynamic behaviour of a mesomorphic gold nanoparticle covered with a liquid-crystalline compound. Chem. Commun. 2640–2641 (2001).
In, I., Jun, Y. W., Kim, Y. J. & Kim, S. Y. Spontaneous one dimensional arrangement of spherical Au nanoparticles with liquid crystal ligands. Chem. Commun. 800–801 (2005).
Draper, M., Saez, I. M., Cowling, S. J., Gai, P., Heinrich, B., Donnio, B., Guillon, D. & Goodby, J. W. Self-assembly and shape morphology of liquid crystalline gold metamaterials. Adv. Funct. Mater. 21, 1260–1278 (2011).
Wojcik, M., Lewandowski, W., Matraszek, J., Mieczkowski, J., Borysiuk, J., Pociecha, D. & Gorecka, E. Liquid-crystalline phases made of gold nanoparticles. Angew. Chem. Int. Ed. 48, 5167–5169 (2009).
Wojcik, M., Kolpaczynska, M., Pociecha, D., Mieczkowski, J. & Gorecka, E. Multidimensional structures made by gold nanoparticles with shape-adaptive grafting layers. Soft Matter 6, 5397–5400 (2010).
Zeng, X., Liu, F., Fowler, A. G., Ungar, G., Cseh, L., Mehl, G. H. & Macdonald, J. E. Ordered gold nanoarrays: 3D ordered gold strings by coating nanoparticles with mesogens. Adv. Mater. 21, 1746–1750 (2009).
Cseh, L. & Mehl, G. H. The design and investigation of room temperature thermotropic nematic gold nanoparticles. J. Am. Chem. Soc. 128, 13376–13377 (2006).
Cseh, L. & Mehl, G. H. Structure–property relationships in nematic gold nanoparticles. J. Mater. Chem. 17, 311–315 (2007).
Marx, V. M., Girgis, H., Heiney, P. A. & Hegmann, T. Bent-core liquid crystal (LC) decorated gold nanoclusters: synthesis, self-assembly, and effects in mixtures with bent-core LC hosts. J. Mater. Chem. 18, 2983–2994 (2008).
Qi, H. & Hegmann, T. Liquid crystal–gold nanoparticle composites. Liq. Cryst. Today 20, 102–114 (2011).
Kumar, S. & Lakshminarayanan, V. Inclusion of gold nanoparticles into a discotic liquid crystalline matrix. Chem. Commun. 1600–1601 (2004).
Marguet, S., Markovitsi, D., Millie, P., Sigal, H. & Kumar, S. Influence of disorder on electronic excited states: an experimental and numerical study of alkylthiotriphenylene columnar phases. J. Phys. Chem. B 102, 4697–4710 (1998).
Kumar, S. & Manickam, M. Oxidative trimerization of o-dialkoxybenzenes to hexaalkoxytriphenylenes: molybdenum(v) chloride as a novel reagent. J. Chem. Soc. Chem. Commun. 1615–1616 (1997).
Kumar, S., Varshney, S. & Chauhan, D. Room-temperature discotic nematic liquid crystals, Mol. Cryst. Liq. Cryst. 396, 241–250 (2003).
Vijayaraghavan, D. & Kumar, S. Self-assembled superlattices of gold nanoparticles in a discotic liquid. Crystal. Mol. Cryst. Liq. Cryst. 508, 101–114 (2009).
Kumar, S., Pal, S. K., Kumar, P. S. & Lakshminarayanan, V. Novel conducting nanocomposites: synthesis of triphenylene-covered gold nanoparticles and their insertion into a columnar matrix. Soft Matter 3, 896–900 (2007).
Holt, L. A., Bushby, R. J., Evans, S. D., Burgess, A. & Seeley, G. A 106-fold enhancement in the conductivity of a discotic liquid crystal doped with only 1% (w/w) gold nanoparticles. J. Appl. Phys. 103, 063712–063717 (2008).
Shen, Z., Yamada, M. & Miyake, M. Control of stripelike and hexagonal self-assembly of gold nanoparticles by the tuning of interactions between triphenylene ligands. J. Am. Chem. Soc. 129, 14271–14280 (2007).
Kumar, P. S., Pal, S. K., Kumar, S. & Lakshminarayanan, V. Dispersion of thiol stabilized gold nanoparticles in lyotropic liquid crystalline systems. Langmuir 23, 3445–3449 (2007).
Liu, L., Miao, Q. & Liang, G. Quantum dots as multifunctional materials for tumor imaging and therapy. Materials 6, 483–499 (2013).
Shirasaki, Y., Supran, G. J., Bawendi, M. G. & Bulovic, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photon 7, 13–23 (2013).
Kamat, P. V. Boosting the efficiency of quantum dot sensitized solar cells through modulation of interfacial charge transfer. Acc. Chem. Res. 45, 1906–1915 (2012).
Semonin, O. E., Luther, J. M. & Beard, M. C. Quantum dots for next-generation photovoltaics. Mater. Today. 15, 508–515 (2012).
Cushing, B. L., Kolesnichenko, V. L. & O’Connor, C. J. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 104, 3893–3946 (2004).
Rao, C. N. R., Vivekchand, S. R. C., Biswas, K. & Govindaraj, A. Synthesis of inorganic nanomaterials. Dalton Trans. 3728–3749 (2007).
Debasis, B., Lei, Q., Teng-Kuan, T. & Paul, H. Quantum dots and their multimodal applications: a review. Materials 3, 2260–2345 (2010).
Murray, C. B., Norris, D. J. & Bawendi, M. G. Synthesis and characterization of nearly monodisperse CdE (E=sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 115, 8706–8715 (1993).
Karanikolos, G. N., Alexandridis, P. & Mountziaris, T. J. Block copolymer-templated synthesis and organization of semiconductor nanocrystals. Macromol. Symp. 289, 43–51 (2010).
Mirzaei, J., Reznikov, M. & Hegmann, T. Quantum dots as liquid crystal dopants. J. Mater. Chem. 22, 22350–22365 (2012).
Kumar, S. & Sagar, L. K. CdSe quantum dots in a columnar matrix. Chem. Commun. 47, 12182–12184 (2011).
Lili, H., Donghuan, Q., Xi, J., Yanshan, L., Li, W., Junwu, C. & Yong, C. Synthesis of high quality zinc-blende CdSe nanocrystals and their application in hybrid solar cells. Nanotechnology 17, 4736–4742 (2006).
Deschenaux, R., Donnio, B. & Guillon, D. Liquid-crystalline fullerodendrimers. New J. Chem. 31, 1064–1073 (2007).
Manickam, M., Smith, A., Belloni, M., Shelley, E. J., Ashton, P. R., Spencer, N. & Preece, J. A. Introduction of bis-discotic and bis-calamitic mesogens addends to C60. Liq. Cryst. 29, 497–504 (2002).
Bushby, R. J., Hamley, I. W., Liu, Q., Lozman, O. R. & Lydon, J. E. Self-assembled columns of fullerene. J. Mater. Chem. 15, 4429–4434 (2005).
Yang, F., Guo, H., Xie, J., Liu, Z. & Xu, B. Synthesis and mesomorphic property of novel discotic C60-triphenylene derivative. Lett. Org. Chem. 8, 599–602 (2001).
Escosura, A., Martínez-Díaz, M. V., Barbera, J. & Torres, T. Self-organization of phthalocyanine-[60]fullerene dyads in liquid crystals. J. Org. Chem. 73, 1475–1480 (2008).
Geerts, Y. H., Debever, O., Amato, C. & Sergeyev, S. Synthesis of mesogenic phthalocyanine-C60 donor–acceptor dyads designed for molecular heterojunction photovoltaic devices. Beilstein J. Org. Chem. 5, 1–9 (2009).
Ince, M., Martínez-Díaz, M. V., Barbera, J. & Torres, T. Liquid crystalline phthalocyanine–fullerene dyad. J. Mater. Chem. 21, 1531–1536 (2011).
Hayashi, H., Nihashi, W., Umeyama, T., Matano, Y., Seki, S., Shimizu, Y. & Imahori, H. Segregated donor-acceptor columns in liquid crystals that exhibit highly efficient ambipolar charge transport. J. Am. Chem. Soc. 133, 10736–10739 (2011).
Kamei, T., Kato, T., Itoh, E. & Ohta, K. Discotic liquid crystals of transition metal complexes 47: synthesis of phthalocyanine-fullerene dyads showing spontaneous homeotropic alignment. J. Porphyrins Phthalocyanines 16, 1261–1275 (2012).
Jurow, M. J., Hageman, B. A., DiMasi, E., Nam, C.-Y., Pabon, C., Blackc, C. T. & Drain, C. M. Controlling morphology and molecular packing of alkane substituted phthalocyanine blend bulk heterojunction solar cells. J. Mater. Chem. A 1, 1557–1565 (2013).
Dao, Q. D., Hori, T., Fukumura, K., Masuda, T., Kamikado, T., Fujii, A., Shimizu, Y. & Ozaki, M. Efficiency enhancement in mesogenic-phthalocyanine-based solar cells with processing additives. Appl. Phys. Lett. 101, 263301 (2012).
Sun, Q., Dai, L., Zhou, X., Li, L. & Li, Q. Bilayer- and bulk-heterojunction solar cells using liquid crystalline porphyrins as donors by solution processing. Appl. Phys. Lett. 91, 253505 (2007).
Wang, C.-L., Zhang, W.-B., Hsu, C.-H., Sun, H.-J., Van Horn, R. M., Tu, Y., Anokhin, D. V., Ivanov, D. A. & Cheng, S. Z. D. A supramolecular structure with an alternating arrangement of donors and acceptors constructed by a trans-di-C60-substituted Zn porphyrin derivative in the solid state. Soft Matter 7, 6135–6143 (2011).
Wang, C.-L., Zhang, W.-B., Sun, H.-J., Van Horn, R. M., Kulkarni, R. R., Tsai, C.-C., Hsu, C.-H., Lotz, B., Gong, X. & Cheng, S. Z. D. A supramolecular ‘double-cable’ structure with a 12944 helix in a columnar porphyrin-C 60 Dyad and its application in polymer solar cells. Adv. Energy Mater. 2, 1375–1382 (2012).
Bisoyi, H. K. & Kumar, S. Carbon-based liquid crystals: art and science. Liq. Cryst. 38, 1427–1449 (2011).
Zheludev, N. I. & Kivshar, Y. S. From metamaterials to metadevices. Nat. Mater. 11, 917–924 (2012).
Kumar, S. & Bisoyi, H. K Aligned carbon nanotubes in the supramolecular order of discotic liquid crystals. Angew. Chem. Int. Ed. 46, 1501–1503 (2007).
Bisoyi, H. K. & Kumar, S. Carbon nanotubes in triphenylene and rufigallol-based room temperature monomeric and polymeric discotic liquid crystals. J. Mater. Chem. 18, 3032–3039 (2008).
Lee, J. J., Yamaguchi, A., Alam, M. A., Yamamoto, Y., Fukushima, T., Kato, K., Takata, M., Fujita, N. & Aida, T. Discotic ionic liquid crystals of triphenylene as dispersants for orienting single-walled carbon nanotubes. Angew. Chem. Int. Ed. 51, 8490–8494 (2012).
Scalia, G., von Bühler, C., Hägele, C., Roth, S., Giesselmann, F. & Lagerwall, J. P. F. Spontaneous macroscopic carbon nanotube alignment via colloidal suspension in hexagonal columnar lyotropic liquid crystals. Soft Matter 4, 570–576 (2008).
Xin, X., Li, H., Kalwarczyk, E., Kelm, A., Marcin, F., Gorecka, E., Pociecha, D. & Holyst, R. Single-walled carbon nanotube/lyotropic liquid crystal hybrid materials fabricated by a phase separation method in the presence of polyelectrolyte. Langmuir 26, 8821–8828 (2010).
Murphy, C. J., Thompson, L. B., Chernak, D. J., Yang, J. A., Sivapalan, S. T., Boulos, S. P., Huang, J., Alkilany, A. M. & Sisco, P. N. Gold nanorod crystal growth: from seed-mediated synthesis to nanoscale sculpting. Curr. Opin. Colloid Interface Sci. 16, 128–134 (2011).
Huang, X., Neretina, S. & El-Sayed, M. A. Gold nanorods: from synthesis and properties to biological and biomedical applications. Adv. Mater. 21, 4880–4910 (2009).
Jana, N. R., Gearheart, L. & Murphy, C. J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv. Mater. 13, 1389–1393 (2001).
Nikoobakht, B. & El-Sayed, M. A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 15, 1957–1962 (2003).
Avinash, B. S., Lakshminarayanan, V., Kumar, S. & Vij, J. K. Gold nanorods embedded discotic nanoribbons. Chem. Commun. 49, 978–980 (2013).
Xiao, S., Tang, J., Beetz, T., Guo, X., Tremblay, N., Siegrist, T., Zhu, Y., Steigerwald, M. & Nuckolls, C. Transferring self-assembled, nanoscale cables into electrical devices. J. Am. Chem. Soc. 128, 10700–10701 (2006).
Wang, H., Xu, X., Kojtari, A. & Ji, H. Triphenylene nano/microwires for sensing nitroaromatics. J. Phys. Chem. C 115, 20091–20096 (2011).
Che, Y., Yang, X., Liu, G., Yu, C., Ji, H., Zuo, J., Zhao, J. & Zang, L. Ultrathin n-type organic nanoribbons with high photoconductivity and application in optoelectronic vapor sensing of explosives. J. Am. Chem. Soc. 132, 5743–5750 (2010).
Wang, H., Xu, X., Li, L., Yang, C. & Ji, H. Optoelectronic property and sensing applications of crystalline nano/microwires of decacyclene. Micro Nano Lett. 6, 763–766 (2011).
Acknowledgements
I thank my colleagues whose work has been summarized in this study. I am grateful to Professor Lakshminarayanan for many helpful discussions and his constant interest in our discotic nanoscience work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kumar, S. Discotic liquid crystal-nanoparticle hybrid systems. NPG Asia Mater 6, e82 (2014). https://doi.org/10.1038/am.2013.75
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/am.2013.75
Keywords
This article is cited by
-
Emerging nanoscience with discotic liquid crystals
Polymer Journal (2021)
-
Atomistic liquid crystalline structures of discotic bent-core-like mesogens formed by hydrogen bonding and interchain interactions
Journal of Molecular Modeling (2020)
-
Thermodynamic study of a plastic columnar discotic material 2, 3, 6, 7, 10, 11-hexabutyloxytriphenylene dispersed with gold nanoparticles under elevated pressure
Journal of Thermal Analysis and Calorimetry (2017)
-
Clicking DNA to gold nanoparticles: poly-adenine-mediated formation of monovalent DNA-gold nanoparticle conjugates with nearly quantitative yield
NPG Asia Materials (2015)
-
Optical, structural, catalytic and electrochemical properties of the Au nanoparticles synthesized using CTAB based gels
Journal of Materials Science: Materials in Electronics (2015)