Abstract
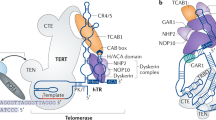
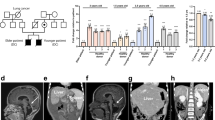
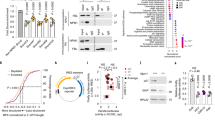
The X-linked form of the human disease dyskeratosis congenita (DKC) is caused by mutations in the gene encoding dyskerin1. Sufferers have defects in highly regenerative tissues such as skin and bone marrow, chromosome instability and a predisposition to develop certain types of malignancy. Dyskerin is a putative pseudouridine synthase, and it has been suggested that DKC may be caused by a defect in ribosomal RNA processing. Here we show that dyskerin is associated not only with H/ACA small nucleolar RNAs2, but also with human telomerase RNA, which contains an H/ACA RNA motif3. Telomerase adds simple sequence repeats to chromosome ends using an internal region of its RNA as a template4, and is required for the indefinite proliferation of primary human cells5. We find that primary fibroblasts and lymphoblasts from DKC-affected males are not detectably deficient in conventional H/ACA small nucleolar RNA accumulation or function; however, DKC cells have a lower level of telomerase RNA, produce lower levels of telomerase activity and have shorter telomeres than matched normal cells. The pathology of DKC is consistent with compromised telomerase function leading to a defect in telomere maintenance, which may limit the proliferative capacity of human somatic cells in epithelia and blood.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Heiss,N. S. et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nature Genet. 19, 32–38 (1998).
Tollervey,D. & Kiss,T. Function and synthesis of small nucleolar RNAs. Curr. Opin. Cell Biol. 9, 337–342 (1997).
Mitchell,J. R., Cheng,J. & Collins,K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol. Cell. Biol. 19, 567–576 (1999).
Yu,G., Bradley,J. D., Attardi,L. D. & Blackburn,E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature 344, 126–132 (1990).
Colgin,L. M. & Reddel,R. R. Telomere maintenance mechanisms and cellular immortalization. Curr. Opin. Genet. Dev. 9, 97–103 (1999).
Nugent,C. I. & Lundblad,V. The telomerase reverse transcriptase: components and regulation. Genes Dev. 12, 1073–1085 (1998).
Lafontaine,D. L. J., Bousquet-Antonelli,C., Henry,Y., Caizergues-Ferrer,M. & Tollervey,D. The box H + ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase. Genes Dev. 12, 527–537 (1998).
Watkins,N. J. et al. Cbf5p, a potential pseudouridine synthase, and Nhp2p, a putative RNA-binding protein, are present together with Gar1p in all H BOX/ACA-motif snoRNPs and constitute a common bipartite structure. RNA 4, 1549–1568 (1998).
Meier,U. T. & Blobel,G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J. Cell Biol. 127, 1505–1514 (1994).
Feng,J. et al. The RNA component of human telomerase. Science 269, 1236–1241 (1995).
Luzzatto,L. & Karadimitris,A. Dyskeratosis and ribosomal rebellion. Nature Genet. 19, 6–7 (1998).
Bryan,T. M., Englezou,A., Gupta,J., Bacchetti,S. & Reddel,R. R. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 14, 4240–4248 (1995).
Bakin,A. & Ofengand,J. Four newly located pseudouridylate residues in Escherichia coli 23S ribosomal RNA are all at the peptidyltransferase center: analysis by the application of a new sequencing technique. Biochemistry 32, 9754–9762 (1993).
Ni,J., Tien,A. & Fournier,M. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell 89, 565–573 (1997).
Ganot,P., Bortolin, M.-L. & Kiss,T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell 89, 799–809 (1997).
Hastie,N. D. et al. Telomere reduction in human colorectal carcinoma and with ageing. Nature 346, 866–868 (1990).
Harley,C. B., Futcher,A. B. & Greider,C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
Counter,C. M., Botelho,F. M., Wang,P., Harley,C. B. & Bacchetti,S. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein–Barr virus-transformed human B lymphocytes. J. Virol. 68, 3410–3414 (1994).
Tahara,H. et al. Abnormal telomere dynamics of B-lymphoblastoid cell strains from Werner's syndrome patients transformed by Epstein–Barr virus. Oncogene 15, 1911–1920 (1997).
Drachtman,R. A. & Alter,B. P. Dyskeratosis congenita: clinical features and genetic heterogeneity. J. Pediat Hematol. Oncol. 14, 297–304 (1992).
Dokal,I. Dyskeratosis congenita: an inherited bone marrow failute syndrome. Br. J. Haematol. 92, 775–779 (1996).
Marsh,J. C. W. et al. “Stem cell” origin of the hematopoietic defect in dyskeratosis congenita. Blood 79, 3138–3144 (1992).
Dokal,I. et al. Dyskeratosis congenita fibroblasts are abnormal and have unbalanced chromosome rearrangements. Blood 80, 3090–3096 (1992).
Sirinavin,C. & Trowbridge,A. A. Dyskeratosis congenita: clinical features and genetic aspects. J. Med. Genet. 12, 339–354 (1975).
Trowbridge,A. A., Sirinavin,C. & Linman,J. W. Dyskeratosis congenita: hematologic evaluation of a sibship and review of the literature. Am. J. Hematol. 3, 143–152 (1977).
Kim,N. W. et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2014 (1994).
Prowse,K. R. & Greider,C. W. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc. Natl. Acad. Sci. USA 92, 4818–4822 (1995).
Acknowledgements
We thank A. Fischer for expert tissue culture assistance; Amgen and the Weinberg laboratory for their hTERT expression constructs; and D. Rio, M. Botchan, J. Rine and members of the Collins laboratory for comments on the manuscript. J.R.M. is a predoctoral fellow of the N.S.F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitchell, J., Wood, E. & Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 (1999). https://doi.org/10.1038/990141
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/990141
This article is cited by
-
Lack of telomerase reduces cancer incidence and increases lifespan of zebrafish tp53M214K mutants
Scientific Reports (2024)
-
Methods that shaped telomerase research
Biogerontology (2024)
-
Telomere length and cancer risk: finding Goldilocks
Biogerontology (2024)
-
Bioinformatic analysis of the effect of SNPs in the pig TERT gene on the structural and functional characteristics of the enzyme to develop new genetic markers of productivity traits
BMC Genomics (2023)
-
Dyskerin and telomerase RNA component are sex-differentially associated with outcomes and Sunitinib response in patients with clear cell renal cell carcinoma
Biology of Sex Differences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.