Abstract
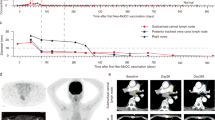
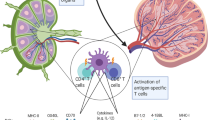
We have developed an individualized melanoma vaccine based on transfection of autologous dendritic cells (DCs) with autologous tumor-mRNA. Dendritic cells loaded with complete tumor-mRNA may generate an immune response against a broad repertoire of antigens, including unique patient-specific antigens. The purpose of the present phase I/II trial was to evaluate the feasibility and safety of the vaccine, and the ability of the DCs to elicit T-cell responses in melanoma patients. Further, we compared intradermal (i.d.) and intranodal (i.n.) vaccine administration. Twenty-two patients with advanced malignant melanoma were included, each receiving four weekly vaccines. Monocyte-derived DCs were transfected with tumor-mRNA by electroporation, matured and cryopreserved. We obtained successful vaccine production for all patients elected. No serious adverse effects were observed. A vaccine-specific immune response was demonstrated in 9/19 patients evaluable by T-cell assays (T-cell proliferation/interferon-γ ELISPOT) and in 8/18 patients evaluable by delayed-type hypersensitivity (DTH) reaction. The response was demonstrated in 7/10 patients vaccinated intradermally and in 3/12 patients vaccinated intranodally. We conclude that immuno-gene-therapy with the described DC-vaccine is feasible and safe, and that the vaccine can elicit in vivo T-cell responses against antigens encoded by the transfected tumor-mRNA. The response rates do not suggest an advantage in applying i.n. vaccination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Thompson JF, Scolyer RA, Kefford RF . Cutaneous melanoma. Lancet 2005; 365: 687–701.
Eigentler TK, Caroli UM, Radny P, Garbe C . Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol 2003; 4: 748–759.
Clemente CG, Mihm Jr MC, Bufalino R, Zurrida S, Collini P, Cascinelli N . Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996; 77: 1303–1310.
Parmiani G, Castelli C, Rivoltini L, Casati C, Tully GA, Novellino L et al. Immunotherapy of melanoma. Semin Cancer Biol 2003; 13: 391–400.
Marchand M, Weynants P, Rankin E, Arienti F, Belli F, Parmiani G et al. Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3. Int J Cancer 1995; 63: 883–885.
Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 1998; 4: 321–327.
Wang F, Bade E, Kuniyoshi C, Spears L, Jeffery G, Marty V et al. Phase I trial of a MART-1 peptide vaccine with incomplete Freund's adjuvant for resected high-risk melanoma. Clin Cancer Res 1999; 5: 2756–2765.
Bettinotti MP, Panelli MC, Ruppe E, Mocellin S, Phan GQ, White DE et al. Clinical and immunological evaluation of patients with metastatic melanoma undergoing immunization with the HLA-Cw*0702-associated epitope MAGE-A12:170-178. Int J Cancer 2003; 105: 210–216.
Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 2005; 115: 739–746.
Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res 2001; 61: 6451–6458.
Mu LJ, Kyte JA, Kvalheim G, Aamdal S, Dueland S, Hauser M et al. Immunotherapy with allotumor mRNA-transfected dendritic cells in androgen-resistant prostate cancer patients. Br J Cancer 2005; 93: 749–756.
Salcedo M, Bercovici N, Taylor N, Vereecken P, Massicard S, Duriau D et al. Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother 2006; 55: 819–829.
Srivastava PK . Do human cancers express shared protective antigens? Or the necessity of remembrance of things past. Semin Immunol 1996; 8: 295–302.
Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E . Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res 2000; 60: 1028–1034.
Anichini A, Mortarini R, Maccalli C, Squarcina P, Fleischhauer K, Mascheroni L et al. Cytotoxic T cells directed to tumor antigens not expressed on normal melanocytes dominate HLA-A2.1-restricted immune repertoire to melanoma. J Immunol 1996; 156: 208–217.
Slingluff CL . Targeting unique tumor antigens and modulating the cytokine environment may improve immunotherapy for tumors with immune escape mechanisms. Cancer Immunol Immunother 1999; 48: 371–373.
Banchereau J, Steinman RM . Dendritic cells and the control of immunity. Nature 1998; 392: 245–252.
Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998; 4: 328–332.
Schuler G, Schuler-Thurner B, Steinman RM . The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol 2003; 15: 138–147.
Tuyaerts S, Michiels A, Corthals J, Bonehill A, Heirman C, de Greef C et al. Induction of influenza matrix protein 1 and melanA-specific T lymphocytes in vitro using mRNA-electroporated dendritic cells. Cancer Gene Ther 2003; 10: 696–706.
Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W . Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood 2000; 96: 3102–3108.
Thurner B, Haendle I, Röder C, Dieckmann D, Keikavoussi P, Jonuleit H et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med 1999; 190: 1669–1678.
Saebøe-Larssen S, Fossberg E, Gaudernack G . mRNA-based electrotransfection of human dendritic cells and induction of cytotoxic T lymphocyte responses against the telomerase catalytic subunit (hTERT). J Immunol Methods 2002; 259: 191–203.
Mu LJ, Gaudernack G, Saebøe-Larssen S, Hammerstad H, Tierens A, Kvalheim G . A protocol for generation of clinical grade mRNA-transfected monocyte-derived dendritic cells for cancer vaccines. Scand J Immunol 2003; 58: 578–586.
Kyte JA, Kvalheim G, Aamdal S, Saebøe-Larssen S, Gaudernack G . Preclinical full-scale evaluation of dendritic cells transfected with autologous tumor-mRNA for melanoma vaccination. Cancer Gene Ther 2005; 12: 579–591.
Nair SK, Morse M, Boczkowski D, Cumming RI, Vasovic L, Gilboa E et al. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg 2002; 235: 540–549.
Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res 2003; 63: 2127–2133.
Caruso DA, Orme LM, Neale AM, Radcliff FJ, Amor GM, Maixner W et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro-oncol 2004; 6: 236–246.
Caruso DA, Orme LM, Amor GM, Neale AM, Radcliff FJ, Downie P et al. Results of a Phase I study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children with Stage 4 neuroblastoma. Cancer 2005; 103: 1280–1291.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216.
Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP et al. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res 2002; 8: 1021–1032.
Vilella R, Benítez D, Milà J, Lozano M, Vilana R, Pomes J et al. Pilot study of treatment of biochemotherapy-refractory stage IV melanoma patients with autologous dendritic cells pulsed with a heterologous melanoma cell line lysate. Cancer Immunol Immunother 2004; 53: 651–658.
Di Nicola M, Carlo-Stella C, Mortarini R, Baldassari P, Guidetti A, Gallino GF et al. Boosting T cell-mediated immunity to tyrosinase by vaccinia virus-transduced, CD34(+)-derived dendritic cell vaccination: a phase I trial in metastatic melanoma. Clin Cancer Res 2004; 10: 5381–5390.
Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J . Autologous dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol 2004; 22: 2808–2815.
Nussenzweig MC, Steinman RM . Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med 1980; 151: 1196–1212.
Scheinecker C, Machold KP, Majdic O, Höcker P, Knapp W, Smolen JS . Initiation of the autologous mixed lymphocyte reaction requires the expression of costimulatory molecules B7-1 and B7-2 on human peripheral blood dendritic cells. J Immunol 1998; 161: 3966–3973.
Wadhwa M, Skog AL, Bird C, Ragnhammar P, Lilljefors M, Gaines-Das R et al. Immunogenicity of granulocyte–macrophage colony-stimulating factor (GM–CSF) products in patients undergoing combination therapy with GM–CSF. Clin Cancer Res 1999; 5: 1353–1361.
Figdor CG, De Vries IJ, Lesterhuis WJ, Melief CJ . Dendritic cell immunotherapy: mapping the way. Nat Med 2004; 10: 475–480.
De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res 2003; 63: 12–17.
Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK . Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res 1999; 59: 56–58.
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ et al. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18: 767–811.
O'Neill DW, Adams S, Bhardwaj N . Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood 2004; 104: 2235–2246.
Rollins BJ . Chemokines. Blood 1997; 90: 909–928.
Balkwill F . Cancer and the chemokine network. Nat Rev Cancer 2004; 4: 540–550.
Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res 2003; 63: 7451–7461.
Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K et al. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 2001; 92: 1085–1091.
Nesbit M, Schaider H, Miller TH, Herlyn M . Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol 2001; 166: 6483–6490.
Martin PJ, Pei J, Gooley T, Anasetti C, Appelbaum FR, Deeg J et al. Evaluation of a CD25-specific immunotoxin for prevention of graft-versus-host disease after unrelated marrow transplantation. Biol Blood Marrow Transplant 2004; 10: 552–560.
Frankel AE, Powell BL, Lilly MB . Diphtheria toxin conjugate therapy of cancer. Cancer Chemother Biol Response Modif 2002; 20: 301–313.
Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 2005; 106: 2018–2025.
Acknowledgements
This work was supported by the Norwegian Ministry of Health (Gene Therapy Grant) and the Norwegian Cancer Society. We thank Mrs Anne Brunsvig, Ms Inger-Lise Haakensen, Mr Sigurd Borgen and Ms Turid Kirsti Hønnåshagen for excellent work on DC generation, and the research nurses Kristin Øwre and Lone Hegg for excellent patient care. We also thank Dr Carrie Grimsrud for helpful work on total RNA extraction and Professor Olav Kaalhus for valuable advice on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kyte, J., Mu, L., Aamdal, S. et al. Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Ther 13, 905–918 (2006). https://doi.org/10.1038/sj.cgt.7700961
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700961
Keywords
This article is cited by
-
Advancing personalized medicine in brain cancer: exploring the role of mRNA vaccines
Journal of Translational Medicine (2023)
-
mRNA therapeutics in cancer immunotherapy
Molecular Cancer (2021)
-
Antitumour dendritic cell vaccination in a priming and boosting approach
Nature Reviews Drug Discovery (2020)
-
Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive prostate cancer
Cancer Immunology, Immunotherapy (2017)
-
In situ dendritic cell vaccination for the treatment of glioma and literature review
Tumor Biology (2016)