Abstract
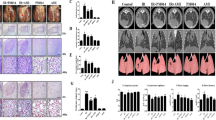
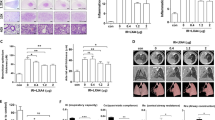
To investigate whether radiation-induced pneumonitis in the mouse-irradiated lung could be prevented by recombinant adenovirus-mediated soluble transforming growth factor-beta (TGF-β) type II receptor gene therapy. Radiation fibrosis-prone mice (C57BL/6J) were randomly divided into four groups consisting of a (1) control group (sham-irradiated); (2) radiation (RT)-alone group; (3) RT+AdCMVsTβR group and (4) RT+AdCMVluc group. The RT-alone and sham-irradiated mice were killed at several time points after thoracic irradiation with a single dose of 9 Gy, and then the TGF-β1 concentrations in serum and broncho-alveolar lavage fluid (BALF) were quantified by enzyme-linked immunosorbent assay (ELISA). We used an adenoviral vector expressing a soluble TGF-β type II receptor (AdCMVsTβR), which can bind to TGF-β and then block the TGF-β receptor-mediated signal transduction. The C57BL/6J mice were intraperitoneally (i.p.) injected with either 5 × 108 plaque-forming units of AdCMVsTβR or AdCMVluc, a control adenovirus-expressing luciferase, a week preceding and a week following the X-ray thoracic irradiation. Four weeks after irradiation, the mice were killed and the concentration of TGF-β1 in the serum and BALF were then measured using ELISA and the lung tissue specimens were examined histopathologically. Following thoracic irradiation with a single dose of 9 Gy, radiation-induced TGF-β1 release in the serum reached the first peak concentration at 12 h and then declined. It reached a maximal value at 2 weeks after irradiation. In the BALF, the TGF-β1 concentration was appreciable within the first hour and thereafter declined. It reached a maximal value at 3 days after irradiation. A one-time i.p. injection of AdCMVsTβR 1 week before irradiation could not completely suppress the two peaks of the radiation-induced TGF-β1 increase, whereas an injection a week preceding and a week following thoracic irradiation was able to suppress those two peaks thoroughly. The TGF-β1 was completely suppressed in the AdCMVsTβR-treated mouse serum and BALF; however, no statistical difference was observed in the serum and BALF between the AdCMVluc-infected mice and the control mice at 4 weeks after irradiation (P<0.05). A histopathological examination showed only mild radiation pneumonitis in the irradiated lungs of AdCMVsTβR-treated mice in comparison to the AdCMVluc-infected and RT-alone mice. Our results demonstrated that TGF-β1 plays an important role in radiation pneumonitis, thus suggesting that the adenovirus-mediated overexpression in soluble TGF-β type II receptor gene therapy may be a potentially feasible and effective strategy for the prevention of radiation pneumonitis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Abratt RP, Morgan GW . Lung toxicity following chest irradiation in patients with lung cancer. Lung Cancer 2002; 35: 103–109.
McDonald S, Rubin P, Phillips TL, Marks LB . Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systems. Int J Radiat Oncol Biol Phys 1995; 31: 1187–1203.
Shapiro SJ, Shapiro SD, Mill WB, Campbell EJ . Prospective study of long-term pulmonary manifestations of mantle irradiation. Int J Radiat Oncol Biol Phys 1990; 19: 707–714.
Roach III M, Gandara DR, Yuo HS, Swift PS, Kroll S, Shrieve DC et al. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol 1995; 13: 2606–2612.
Martel MK, Ten Haken RK, Hazuka MB, Turrisi AT, Fraass BA, Lichter AS . Dose-volume histogram and 3-D treatment planning evaluation of patients with pneumonitis. Int J Radiat Oncol Biol Phys 1994; 28: 575–581.
Lingos TI, Recht A, Vicini F, Abner A, Silver B, Harris JR . Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys 1991; 21: 355–360.
Vujaskovic Z, Marks LB, Anscher MS . The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol 2000; 10: 296–307.
Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN . A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys 1995; 33: 99–109.
Chen Y, Williams J, Ding I, Hernady E, Liu W, Smudzin T et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol 2002; 12: 26–33.
Massague J, Attisano L, Wrana JL . The TGF-beta family and its composite receptors. Trends Cell Biol 1994; 4: 172–178.
Border WA, Noble NA . Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994; 331: 1286–1292.
Massague J . TGF-beta signal transduction. Annu Rev Biochem 1998; 67: 753–791.
Oberhammer FA, Pavelka M, Sharma S, Tiefenbacher R, Purchio AF, Bursch W et al. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci USA 1992; 89: 5408–5412.
Kitamura S, Maeshima Y, Sugaya T, Sugiyama H, Yamasaki Y, Makino H . Transforming growth factor-beta 1 induces vascular endothelial growth factor expression in murine proximal tubular epithelial cells. Nephron Exp Nephrol 2003; 95: e79–e86.
Beck C, Schreiber H, Rowley D . Role of TGF-beta in immune-evasion of cancer. Microsc Res Tech 2001; 52: 387–395.
Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N . The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol 2004; 172: 1848–1855.
Flaumenhaft R, Abe M, Mignatti P, Rifkin DB . Basic fibroblast growth factor-induced activation of latent transforming growth factor beta in endothelial cells: regulation of plasminogen activator activity. J Cell Biol 1992; 118: 901.
Riley PA . Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 1994; 65: 27–33.
Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys 2001; 50: 851–855.
Letterio JJ, Roberts AB . Regulation of immune responses by TGF-beta. Annu Rev Immunol 1998; 16: 137–161.
Ashcroft GS . Bidirectional regulation of macrophage function by TGF-beta. Microbes Infect 1999; 1: 1275–1282.
Massague J . The transforming growth factor-beta family. Annu Rev Cell Biol 1990; 6: 597–641.
Wang XF, Lin HY, Ng-Eaton E, Downward J, Lodish HF, Weinberg RA . Expression cloning and characterization of the TGF-beta type III receptor. Cell 1991; 67: 797–805.
Wrana JL, Attisano L, Wieser R, Ventura F, Massague J . Mechanism of activation of the TGF-beta receptor. Nature 1994; 370: 341–347.
Yamamoto H, Atsuchi N, Tanaka H, Ogawa W, Abe M, Takeshita A et al. Separate roles for H-Ras and Rac in signaling by transforming growth factor (TGF)-beta. H-Ras is essential for activation of MAP kinase, partially required for transcriptional activation by TGF-beta, but not required for signaling of growth suppression by TGF-beta. Eur J Biochem 1999; 264: 110–119.
Derynck R, Zhang YE . Smad-dependent and Smad-independent pathway in TGF-beta family signaling. Nature 2003; 425: 577–584.
Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell 1992; 71: 1003–1014.
Carcamo J, Weis FM, Ventura F, Wieser R, Wrana JL, Attisano L et al. Type I receptors specify growth-inhibitory and transcriptional responses to transforming growth factor beta and activin. Mol Cell Biol 1994; 14: 3810–3821.
Bhushan A, Lin HY, Lodish HF, Kintner CR . The transforming growth factor beta type II receptor can replace the activin type II receptor in inducing mesoderm. Mol Cell Biol 1994; 14: 4280–4285.
Anscher MS, Murase T, Prescott DM, Marks LB, Reisenbichler H, Bentel GC et al. Changes in plasma TGF beta levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int J Radiat Oncol Biol Phys 1994; 30: 671–676.
Vujaskovic Z, Down JD, van Waarde MA, van Assen AJ, Szabo BG, Konings AW . Plasma TGF beta level in rats after hemithoracic irradiation. Radiother Oncol 1997; 44: 41–43.
Anscher MS, Kong FM, Andrews K, Clough R, Marks LB, Bentel G et al. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys 1998; 41: 1029–1035.
Anscher MS, Kong FM, Marks LB, Bentel GC, Jirtle RL . Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys 1997; 37: 253–258.
Wang Q, Wang Y, Hyde DM, Gotwals PJ, Koteliansky VE, Ryan ST et al. Reduction of bleomycin induced fibrosis by transforming growth factor beta soluble receptor in hamsters. Thorax 1999; 54: 805–812.
Zheng H, Wang J, Koteliansky VE, Gotwals PJ, Hauer-Jensen M . Recombinant soluble transforming growth factor beta type II receptor ameliorates radiation enteropathy in mice. Gastroenterology 2000; 119: 1286–1296.
Haviv YS, Takayama K, Nagi PA, Tousson A, Cook W, Wang M et al. Modulation of renal glomerular disease using remote delivery of adenoviral-encoded soluble type II TGF-beta receptor fusion molecule. J Gene Med 2003; 5: 839–851.
He T-C, Zhou S, DaCosta LT, Yu J, Kinzler KW, Vogelstein B . A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514.
Liu YC, Kawagishi M, Kameda R, Ohashi H . Characterization of a fusion protein composed of the extracellular domain of c-kit and the Fc region of human IgG expressed in a baculovirus system. Biochem Biophys Res Commun 1993; 197: 1094–1102.
Isaka Y, Akagi Y, Ando Y, Tsujie M, Sudo T, Ohno N et al. Gene therapy by transforming growth factor-β receptor-IgG Fc chimera suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int 1999; 55: 465–475.
Ueno H, Sakamoto T, Nakamura T, Qi Z, Astuchi N, Takeshita A et al. A soluble transforming growth factor β receptor expressed in muscle prevents liver fibrogenesis and dysfunction in rats. Hum Gene Ther 2000; 11: 33–42.
Oshima Y, Sakamoto T, Hisatomi T, Tsutsumi C, Ueno H, Ishibashi T . Gene transfer of soluble TGF-β type II receptor inhibits experimental proliferative vitreoretinopathy. Gene Therapy 2002; 9: 1214–1220.
Sakamoto T, Ueno H, Sonoda K, Hisatomi T, Shimizu K, Ohashi H et al. Blockade of TGF-beta by in vivo gene transfer of a soluble TGF-β type II receptor in the muscle inhibits corneal opacification, edema and angiogenesis. Gene Therapy 2000; 7: 1915–1924.
Ashcroft T, Simpson JM, Timbrell V . Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988; 41: 467–470.
Kucich U, Rosenbloom JC, Shen G, Abrams WR, Hamilton AD, Sebti SM et al. TGF-β1 stimulation of fibronectin transcription in cultured human lung fibroblasts requires active geranylgeranyl transferase I, phosphatidylcholine-specific phospholipase C, protein kinase C-delta, and p38, but not erk1/erk2. Arch Biochem Biophys 2000; 374: 313–324.
Roberts CJ, Birkenmeier TM, McQuillan JJ, Akiyama SK, Yamada SS, Chen WT et al. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem 1988; 263: 4586–4592.
Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A et al. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys 2000; 47: 1033–1042.
Burger A, Loffler H, Bamberg M, Rodemann HP . Molecular and cellular basis of radiation fibrosis. Int J Radiat Biol 1998; 73: 401–408.
Anscher MS, Kong FM, Jirtle RL . The relevance of transforming growth factor beta 1 in pulmonary injury after radiation therapy. Lung Cancer 1998; 19: 109–120.
Martin M, Lefaix J, Delanian S . TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys 2000; 47: 277–290.
Novakova-Jiresova A, Van Gameren MM, Coppes RP, Kampinga HH, Groen HJ . Transforming growth factor-beta plasma dynamics and post-irradiation lung injury in lung cancer patients. Radiother Oncol 2004; 71: 183–189.
Vujaskovic Z, Groen HJ . TGF-beta, radiation-induced pulmonary injury and lung cancer. Int J Radiat Biol 2000; 76: 511–516.
Rubin P, Shapiro DL, Finklestein JN, Penney DP . The early release of surfactant following lung irradiation of alveolar type II cells. Int J Radiat Oncol Biol Phys 1980; 6: 75–77.
Worgall S, Wolff G, Falck-Pedersen E, Crystal RG . Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther 1997; 8: 37–44.
Alemany R, Suzuki K, Curiel DT . Blood clearance rates of adenovirus type 5 in mice. J Gen Virol 2000; 81: 2605–2609.
Rabbani ZN, Anscher MS, Zhang X, Chen L, Samulski TV, Li CY et al. Soluble TGFbeta type II receptor gene therapy ameliorates acute radiation-induced pulmonary injury in rats. Int J Radiat Oncol Biol Phys 2003; 57: 563–572.
Nishioka A, Ogawa Y, Mima T, Jin YJ, Sonobe H, Kariya S et al. Histopathologic amelioration of fibroproliferative change in rat irradiated lung using soluble transforming growth factor-beta (TGF-beta) receptor mediated by adenoviral vector. Int J Radiat Oncol Biol Phys 2004; 58: 1235–1241.
Zhang K, Flanders KC, Phan SH . Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. Am J Pathol 1995; 147: 352–361.
Hu B, Wu Z, Phan SH . Smad3 mediates transforming growth factor β-induced α-smooth muscle actin expression. Am J Respir Cell Mol Biol 2003; 29: 397–404.
Badie B, Goh CS, Klaver J, Herweijer H, Boothman DA . Combined radiation and p53 gene therapy of malignant glioma cells. Cancer Gene Ther 1999; 6: 155–162.
Sasaki R, Shirakawa T, Zhang ZJ, Tamekane A, Matsumoto A, Sugimura K et al. Additional gene therapy with Ad5CMV-p53 enhanced the efficacy of radiotherapy in human prostate cancer cells. Int J Radiat Oncol Biol Phys 2001; 51: 1336–1345.
Acknowledgements
We thank Dr Mizuho Yamada for her valuable help and technical assistance in the animal experiment. We also appreiciate the assistance of Ms Chie Kihara for her help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haiping, Z., Takayama, K., Uchino, J. et al. Prevention of radiation-induced pneumonitis by recombinant adenovirus-mediated transferring of soluble TGF-β type II receptor gene. Cancer Gene Ther 13, 864–872 (2006). https://doi.org/10.1038/sj.cgt.7700959
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700959
Keywords
This article is cited by
-
Gene-modified Mesenchymal Stem Cells Protect Against Radiation-induced Lung Injury
Molecular Therapy (2013)