Abstract
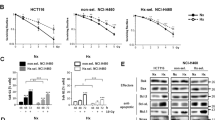
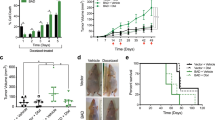
Radiation-induced apoptosis is thought to underlie the toxicity of radiation to normal tissues as well as to cancer cells. We hypothesized that specific ectopic overexpression of the antiapoptotic molecule Bcl-2 in normal cells would inhibit radiation-induced apoptosis and thereby reduce radiation-induced toxicity in normal cells. To express Bcl-2 specifically in normal cells (which have wild-type (wt) p53) but not in cancer cells (which often have mutated p53), we constructed a Bcl-2 expression plasmid (PG13-Bcl-2) with a minimal promoter regulated by multiple wt p53 DNA-binding sites and found that the presence of wt p53 protein strongly upregulated Bcl-2 expression through this plasmid. Transfection of NIH 3T3 fibroblasts, which express wt p53, with PG13-Bcl-2 increased cell survival and reduced apoptosis; however, transfection of MDA-MB-231 breast cancer cells, which have mutated p53, did not affect survival and apoptosis of those cells. These results indicate that irradiation of normal cells rapidly upregulates the expression of wt p53, which binds to the p53 binding sequence of the PG13-Bcl-2 plasmid and increases the transcriptional activity of Bcl-2. Ectopic expression of Bcl-2 reduced radiation-induced apoptosis only in normal cells (not in cancer cells). Bcl-2 expression was detected in the lung from mice injected via a tail vein with LPD-PG13-Bcl-2 or LPD-CMV-Bcl-2, but did not in the lung from mice treated with DOTAP or LPD-PG13-mock. This novel approach to inhibiting radiation-induced apoptosis in normal cells may allow such cells to be protected from radiation-induced toxicity. Further preclinical in vivo studies are needed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001; 293: 293–297.
Pena LA, Fuks Z, Kolesnick RN . Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 2000; 60: 321–327.
Deng W, Viar MJ, Johnson LR . Polyamine depletion inhibits irradiation-induced apoptosis in intestinal epithelia. Am J Physiol Gastrointest Liver Physiol 2005; 289: G599–G606.
Reed JC . Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994; 124: 1–6.
Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC . Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res 1993; 53: 4701–4714.
Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ . bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 1991; 67: 879–888.
Kyprianou N, King ED, Bradbury D, Rhee JG . bcl-2 over-expression delays radiation-induced apoptosis without affecting the clonogenic survival of human prostate cancer cells. Int J Cancer 1997; 70: 341–348.
Levine B, Huang Q, Isaacs JT, Reed JC, Griffin DE, Hardwick JM . Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature 1993; 361: 739–742.
Huang A, Fone PD, Gandour-Edwards R, White RW, Low RK . Immunohistochemical analysis of BCL-2 protein expression in renal cell carcinoma. J Urol 1999; 162: 610–613.
Berns EM, Foekens JA, Vossen R, Look MP, Devilee P, Henzen-Logmans SC et al. Complete sequencing of TP53 predicts poor response to systemic therapy of advanced breast cancer. Cancer Res 2000; 60: 2155–2162.
Alsner J, Yilmaz M, Guldberg P, Hansen LL, Overgaard J . Heterogeneity in the clinical phenotype of TP53 mutations in breast cancer patients. Clin Cancer Res 2000; 6: 3923–3931.
Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sorlie T et al. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res 1994; 22: 3551–3555.
Kern SE, Pietenpol JA, Thiagalingam S, Seymour A, Kinzler KW, Vogelstein B . Oncogenic forms of p53 inhibit p53-regulated gene expression. Science 1992; 256: 827–830.
Mosmann T . Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63.
Rehemtulla A, Hamilton AC, Taneja N, Fridman J, Juan TS, Maybaum J et al. A caspase-resistant form of Bcl-X(L), but not wild type Bcl-X(L), promotes clonogenic survival after ionizing radiation. Neoplasia 1999; 1: 63–70.
Rudner J, Belka C, Marini P, Wagner RJ, Faltin H, Lepple-Wienhues A et al. Radiation sensitivity and apoptosis in human lymphoma cells. Int J Radiat Biol 2001; 77: 1–11.
Froesch BA, Aime-Sempe C, Leber B, Andrews D, Reed JC . Inhibition of p53 transcriptional activity by Bcl-2 requires its membrane-anchoring domain. J Biol Chem 1999; 274: 6469–6475.
Mirkovic N, Voehringer DW, Story MD, McConkey DJ, McDonnell TJ, Meyn RE . Resistance to radiation-induced apoptosis in Bcl-2-expressing cells is reversed by depleting cellular thiols. Oncogene 1997; 15: 1461–1470.
Brustugun OT, Fladmark KE, Doskeland SO, Orrenius S, Zhivotovsky B . Apoptosis induced by microinjection of cytochrome c is caspase-dependent and is inhibited by Bcl-2. Cell Death Differ 1998; 5: 660–668.
Oltvai ZN, Milliman CL, Korsmeyer SJ . Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993; 74: 609–619.
Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 1998; 281: 2027–2031.
Tepper AD, de Vries E, van Blitterswijk WJ, Borst J . Ordering of ceramide formation, caspase activation, and mitochondrial changes during CD95- and DNA damage-induced apoptosis. J Clin Invest 1999; 103: 971–978.
Strasser A, Harris AW, Jacks T, Cory S . DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell 1994; 79: 329–339.
Story MD, Voehringer DW, Malone CG, Hobbs ML, Meyn RE . Radiation-induced apoptosis in sensitive and resistant cells isolated from a mouse lymphoma. Int J Radiat Biol 1994; 66: 659–668.
Agarwal ML, Agarwal A, Taylor WR, Chernova O, Sharma Y, Stark GR . A p53-dependent S-phase checkpoint helps to protect cells from DNA damage in response to starvation for pyrimidine nucleotides. Proc Natl Acad Sci USA 1998; 95: 14775–14780.
Vousden KH, Lu X . Live or let die: the cell's response to p53. Nat Rev Cancer 2002; 2: 594–604.
Fei P, Bernhard EJ, El-Deiry WS . Tissue-specific induction of p53 targets in vivo. Cancer Res 2002; 62: 7316–7327.
McIlwrath AJ, Vasey PA, Ross GM, Brown R . Cell cycle arrests and radiosensitivity of human tumor cell lines: dependence on wild-type p53 for radiosensitivity. Cancer Res 1994; 54: 3718–3722.
Aldridge DR, Radford IR . Explaining differences in sensitivity to killing by ionizing radiation between human lymphoid cell lines. Cancer Res 1998; 58: 2817–2824.
Hwang JH, Lim SC, Kim YC, Park KO, Ahn SJ, Chung WK . Apoptosis and bcl-2 expression as predictors of survival in radiation-treated non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2001; 50: 13–18.
Gudkov AV, Komarova EA . The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 2003; 3: 117–129.
Ahrendt SA, Hu Y, Buta M, McDermott MP, Benoit N, Yang SC et al. p53 mutations and survival in stage I non-small-cell lung cancer: results of a prospective study. J Natl Cancer Inst 2003; 95: 961–970.
Li S, Huang L . In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther 1997; 4: 891–900.
Li S, Rizzo MA, Bhattacharya S, Huang L . Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery. Gene Ther 1998; 5: 930–937.
Acknowledgements
We thank Christine Wogan of Department of Scientific Publications at MD Anderson Cancer Center for her editorial assistance. Grant support from Congressionally Directed Medical Research Program in Breast Cancer, US Department of Defense. DAMD17-01-1-0304 03.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Itamochi, H., Yamasaki, F., Sudo, T. et al. Reduction of radiation-induced apoptosis by specific expression of Bcl-2 in normal cells. Cancer Gene Ther 13, 451–459 (2006). https://doi.org/10.1038/sj.cgt.7700920
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700920