Abstract
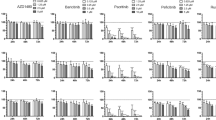
Multiple myeloma (MM) accounts for 10% of hematological malignant disorders. Its refractory nature indicates the necessity of developing novel therapeutic modalities. Since interleukin 6 (IL-6) is one of the major growth factors for MM cells, we expressed suppressor of cytokine signaling-1 (SOCS-1), one of the blockades of IL-6 receptor downstream signaling, to suppress the proliferation of MM cells. Because MM cells are resistant to conventional adenoviral vector infection, we utilized infectivity-enhanced adenoviral vectors with an RGD4C motif in the adenoviral fiber-knob region (RGD-modified vector). In infectivity analysis, RGD-modified vectors were superior to unmodified controls in the majority of the MM cell lines tested. The overexpression of SOCS-1 using infectivity-enhanced adenoviral vectors achieved growth suppression in IL-6-dependent MM cells, but not in the IL-6-independent cells. IL-6-induced STAT3 phosphorylation was suppressed in IL-6-dependent cells, indicating that the signal transduction cascade of the IL-6 receptor signaling was blocked. In aggregate, SOCS-1 overexpression with RGD-modified adenoviral vectors achieved the antiproliferative effect in IL-6-dependent MM cells. These results provide an initial proof-of-principle of the anticancer effect of SOCS-1 expression vector as well as a promise for the future development of therapeutic modality for MM based on this vector.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Jemal A, Thomas A, Murray T, Thun M . Cancer statistics, 2002. CA Cancer J Clin 2002; 52: 23–47.
Bataille R, Harousseau JL . Multiple myeloma. N Engl J Med 1997; 336: 1657–1664.
Rajkumar SV, Gertz MA, Kyle RA, Greipp PR . Current therapy for multiple myeloma. Mayo Clin Proc 2002; 77: 813–822.
Fassas A, Tricot G . Results of high-dose treatment with autologous stem cell support in patients with multiple myeloma. Semin Hematol 2001; 38: 231–242.
Bensinger WI, Maloney D, Storb R . Allogeneic hematopoietic cell transplantation for multiple myeloma. Semin Hematol 2001; 38: 243–249.
Teoh G, Chen L, Urashima M, Tai YT, Celi LA, Chen D et al. Adenovirus vector-based purging of multiple myeloma cells. Blood 1998; 92: 4591–4601.
Russell SJ, Dunbar CE . Gene therapy approaches for multiple myeloma. Semin Hematol 2001; 38: 268–275.
Zhang XG, Klein B, Bataille R . Interleukin-6 is a potent myeloma-cell growth factor in patients with aggressive multiple myeloma. Blood 1989; 74: 11–13.
Borset M, Waage A, Brekke OL, Helseth E . TNF and IL-6 are potent growth factors for OH-2, a novel human myeloma cell line. Eur J Haematol 1994; 53: 31–37.
Nishimoto N, Ogata A, Shima Y, Tani Y, Ogawa H, Nakagawa M et al. Oncostatin M, leukemia inhibitory factor, and interleukin 6 induce the proliferation of human plasmacytoma cells via the common signal transducer, gp130. J Exp Med 1994; 179: 1343–1347.
Tsunenari T, Akamatsu K, Kaiho S, Sato K, Tsuchiya M, Koishihara Y et al. Therapeutic potential of humanized anti-interleukin-6 receptor antibody: antitumor activity in xenograft model of multiple myeloma. Anticancer Res 1996; 16: 2537–2544.
Tsunenari T, Koishihara Y, Nakamura A, Moriya M, Ohkawa H, Goto H et al. New xenograft model of multiple myeloma and efficacy of a humanized antibody against human interleukin-6 receptor. Blood 1997; 90: 2437–2444.
Nishimoto N, Shima Y, Yoshizaki K, Kishimoto T . Myeloma biology and therapy. Present status and future developments. Hematol Oncol Clin N Am 1997; 11: 159–172.
Anderson K . Advances in the biology of multiple myeloma: therapeutic applications. Semin Oncol 1999; 26: 10–22.
Akira S . IL-6-regulated transcription factors. Int J Biochem Cell Biol 1997; 29: 1401–1418.
Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L . Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 1998; 334 (Part 2): 297–314.
Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F . Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003; 374: 1–20.
Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A et al. Structure and function of a new STAT-induced STAT inhibitor. Nature 1997; 387: 924–929.
Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ et al. A family of cytokine-inducible inhibitors of signalling. Nature 1997; 387: 917–921.
Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 1997; 387: 921–924.
Suematsu S, Hibi M, Sugita T, Saito M, Murakami M, Matsusaka T et al. Interleukin 6 (IL-6) and its receptor (IL-6R) in myeloma/plasmacytoma. Curr Top Microbiol Immunol 1990; 166: 13–22.
Suzuki H, Yasukawa K, Saito T, Goitsuka R, Hasegawa A, Ohsugi Y et al. Anti-human interleukin-6 receptor antibody inhibits human myeloma growth in vivo. Eur J Immunol 1992; 22: 1989–1993.
Chauhan D, Uchiyama H, Urashima M, Yamamoto K, Anderson KC . Regulation of interleukin 6 in multiple myeloma and bone marrow stromal cells. Stem Cells 1995; 13 (Suppl 2): 35–39.
Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 2003; 4: 540–545.
Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN et al. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol 2003; 4: 546–550.
Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 2003; 4: 551–556.
Nicholson SE, Willson TA, Farley A, Starr R, Zhang JG, Baca M et al. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J 1999; 18: 375–385.
Curiel DT, Gerritsen WR, Krul MR . Progress in cancer gene therapy. Cancer Gene Ther 2000; 7: 1197–1199.
Sachs M, Rauen K, Ramamurthy M, Dodson JL, De Marzo AM, Putzi MJ et al. Integrin alpha(v) and coxsackie adenovirus receptor expression in clinical bladder cancer. Urology 2002; 60: 531.
You Z, Fischer DC, Tong X, Hasenburg A, Aguilar-Cordova E, Kieback DG . Coxsackievirus-adenovirus receptor expression in ovarian cancer cell lines is associated with increased adenovirus transduction efficiency and transgene expression. Cancer Gene Ther 2001; 8: 168–175.
Gonzalez R, Vereecque R, Wickham TJ, Facon T, Hetuin D, Kovesdi I et al. Transduction of bone marrow cells by the AdZ.F(pK7) modified adenovirus demonstrates preferential gene transfer in myeloma cells. Hum Gene Ther 1999; 10: 2709–2717.
Krasnykh V, Dmitriev I, Navarro JG, Belousova N, Kashentseva E, Xiang J et al. Advanced generation adenoviral vectors possess augmented gene transfer efficiency based upon coxsackie adenovirus receptor-independent cellular entry capacity. Cancer Res 2000; 60: 6784–6787.
Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol 1998; 72: 9706–9713.
Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT . Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol 1998; 72: 1844–1852.
Garcia-Gila M, Cabanas C, Garcia-Pardo A . Analysis of the activation state of alpha4beta1 integrin in human B cell lines derived from myeloma, leukemia or lymphoma. FEBS Lett 1997; 418: 337–340.
Pierschbacher MD, Ruoslahti E . Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984; 309: 30–33.
Okuno Y, Takahashi T, Suzuki A, Fukumoto M, Nakamura K, Fukui H et al. Acquisition of growth autonomy and tumorigenicity by an interleukin 6-dependent human myeloma cell line transfected with interleukin 6 cDNA. Exp Hematol 1992; 20: 395–400.
Matsuda T, Hirano T, Kishimoto T . Establishment of an interleukin 6 (IL 6)/B cell stimulatory factor 2-dependent cell line and preparation of anti-IL 6 monoclonal antibodies. Eur J Immunol 1988; 18: 951–956.
Aarden LA, De Groot ER, Schaap OL, Lansdorp PM . Production of hybridoma growth factor by human monocytes. Eur J Immunol 1987; 17: 1411–1416.
Goto H, Shimazaki C, Tatsumi T, Yamagata N, Fujita N, Tsuchiya M et al. Establishment of a novel myeloma cell line KPMM2 carrying t(3;14)(q21;q32), which proliferates specifically in response to interleukin-6 through an autocrine mechanism. Leukemia 1995; 9: 711–718.
Namba M, Ohtsuki T, Mori M, Togawa A, Wada H, Sugihara T et al. Establishment of five human myeloma cell lines. In vitro Cell Dev Biol 1989; 25: 723–729.
Shimizu S, Takiguchi T, Sugai S, Matsuoka M, Konda S . An established CD4+ T lymphoma cell line derived from a patient with so-called Lennert's lymphoma: possible roles of cytokines in histopathogenesis. Blood 1988; 71: 196–203.
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B . A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514.
Yamamoto M, Curiel DT . Nonreplicating DNA viral vectors for suicide gene therapy: the adenoviral vectors. Methods Mol Med 2004; 90: 61–70.
Narazaki M, Fujimoto M, Matsumoto T, Morita Y, Saito H, Kajita T et al. Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc Natl Acad Sci USA 1998; 95: 13130–13134.
Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 1986; 324: 73–76.
Klein B, Zhang XG, Jourdan M, Portier M, Bataille R . Interleukin-6 is a major myeloma cell growth factor in vitro and in vivo especially in patients with terminal disease. Curr Top Microbiol Immunol 1990; 166: 23–31.
Westendorf JJ, Ahmann GJ, Greipp PR, Witzig TE, Lust JA, Jelinek DF . Establishment and characterization of three myeloma cell lines that demonstrate variable cytokine responses and abilities to produce autocrine interleukin-6. Leukemia 1996; 10: 866–876.
Schwab G, Siegall CB, Aarden LA, Neckers LM, Nordan RP . Characterization of an interleukin-6-mediated autocrine growth loop in the human multiple myeloma cell line, U266. Blood 1991; 77: 587–593.
Hitzler JK, Martinez-Valdez H, Bergsagel DB, Minden MD, Messner HA . Role of interleukin-6 in the proliferation of human multiple myeloma cell lines OCI-My 1 to 7 established from patients with advanced stage of the disease. Blood 1991; 78: 1996–2004.
Tsunenari T, Akamatsu K, Kaiho S, Sato K, Tsuchiya M, Koishihara Y et al. Therapeutic potential of humanized anti-interleukin-6 receptor antibody: antitumor activity in xenograft model of multiple myeloma. Anticancer Res 1996; 16: 2537–2544.
Hirano T, Ishihara K, Hibi M . Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000; 19: 2548–2556.
Minami M, Inoue M, Wei S, Takeda K, Matsumoto M, Kishimoto T et al. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc Natl Acad Sci USA 1996; 93: 3963–3966.
Eling DJ, Johnson PA, Sharma S, Tufaro F, Kipps TJ . Chronic lymphocytic leukemia B cells are highly sensitive to infection by herpes simplex virus-1 via herpesvirus-entry-mediator A. Gene Therapy 2000; 7: 1210–1216.
Wickham TJ, Roelvink PW, Brough DE, Kovesdi I . Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol 1996; 14: 1570–1573.
Wickham TJ, Tzeng E, Shears II LL, Roelvink PW, Li Y, Lee GM et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol 1997; 71: 8221–8229.
Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood 2001; 97: 3746–3754.
Tatsumi T, Shimazaki C, Goto H, Araki S, Sudo Y, Yamagata N et al. Expression of adhesion molecules on myeloma cells. Jpn J Cancer Res 1996; 87: 837–842.
Uchiyama H, Barut BA, Chauhan D, Cannistra SA, Anderson KC . Characterization of adhesion molecules on human myeloma cell lines. Blood 1992; 80: 2306–2314.
Wickham TJ, Mathias P, Cheresh DA, Nemerow GR . Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 1993; 73: 309–319.
Drew M, Barker HF, Ball J, Pearson C, Cook G, Franklin I . Very late antigen (VLA) expression by normal and neoplastic human plasma cells; including an assessment of antibodies submitted to the Vth International Workshop on Leucocyte Differentiation Antigens using human myeloma cell lines. Leuk Res 1996; 20: 619–624.
Ruoslahti E . RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 1996; 12: 697–715.
Krebs DL, Hilton DJ . SOCS proteins: negative regulators of cytokine signaling. Stem Cells 2001; 19: 378–387.
Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T et al. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J 1999; 18: 1309–1320.
He TC, Jiang N, Zhuang H, Wojchowski DM . Erythropoietin-induced recruitment of Shc via a receptor phosphotyrosine-independent, Jak2-associated pathway. J Biol Chem 1995; 270: 11055–11061.
Winston LA, Hunter T . JAK2, Ras, and Raf are required for activation of extracellular signal-regulated kinase/mitogen-activated protein kinase by growth hormone. J Biol Chem 1995; 270: 30837–30840.
Ogata A, Chauhan D, Teoh G, Treon SP, Urashima M, Schlossman RL et al. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol 1997; 159: 2212–2221.
Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 1999; 10: 105–115.
Hideshima T, Nakamura N, Chauhan D, Anderson KC . Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene 2001; 20: 5991–6000.
Ilangumaran S, Ramanathan S, La Rose J, Poussier P, Rottapel R . Suppressor of cytokine signaling 1 regulates IL-15 receptor signaling in CD8+CD44high memory T lymphocytes. J Immunol 2003; 171: 2435–2445.
Naka T, Fujimoto M, Kishimoto T . Negative regulation of cytokine signaling: STAT-induced STAT inhibitor. Trends Biochem Sci 1999; 24: 394–398.
Acknowledgements
This work was supported in part by the National Organization for Rare Disorders, Research Grant Program for Multiple Myeloma to Masato Yamamoto, and by a Grant-in-Aid of the Ministry of Health, Labor and Welfare of Japan to Norihiro Nihsimoto. We thank Dr Victor Krasnykh for providing the Ad backbone plasmid for infectivity-enhanced vectors, and Dr Kazuyuki Yoshizaki and Long Le for excellent advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, M., Nishimoto, N., Davydova, J. et al. Suppressor of cytokine signaling-1 expression by infectivity-enhanced adenoviral vector inhibits IL-6-dependent proliferation of multiple myeloma cells. Cancer Gene Ther 13, 194–202 (2006). https://doi.org/10.1038/sj.cgt.7700873
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700873
Keywords
This article is cited by
-
β-adrenergic modulation of IL-6/gp130 and SOCS-1 in multiple myeloma: therapeutic strategy for stress induced-inflammatory response
memo - Magazine of European Medical Oncology (2024)
-
Suppression of essential pro-inflammatory signaling pathways by natural agents for the therapy of Multiple Myeloma
Phytochemistry Reviews (2014)