Abstract
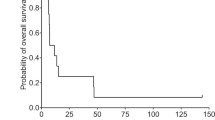
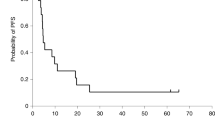
Following our pilot clinical study of combined IL-2/HSV-TK gene therapy for recurrent glioblastoma multiforme (GBM), we extended the protocol to a larger population of patients and evaluated safety, feasibility, and biological activity of treatment. A total of 12 patients received intratumor injection of retroviral vector-producing cells (RVPCs), followed by intravenous ganciclovir (GCV). Treatment was well tolerated with only minor adverse events. Transduction of tumor cells was demonstrated in tumor biopsies. A marked and persistent increase of intratumor and plasma Th1 cytokine levels was demonstrated after RVPC injection. At magnetic resonance imaging evaluation, two patients had a partial response (including a patient showing disappearance of a distant noninjected tumor mass), four had a minor response, four had stable disease, and two had progressive disease. The 6- and 12-month progression-free survival rates were 47 and 14%, respectively. The 6- and 12-month overall survival rates were 58 and 25%, respectively. In conclusion, the results of our clinical protocol of gene therapy for recurrent GBM, based on combined delivery of a suicide and a cytokine gene, demonstrate that intratumor injection of RVPCs was safe, provided effective transduction of the therapeutic genes to target tumor cells, and activated a systemic cytokine cascade, with tumor responses in 50% of cases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Huncharek M, Muscat J . Treatment of recurrent high grade astrocytomas; results of a systematic review of 1415 patients. Anticancer Res. 1998;18:1303–1311.
Evren Keles G, Lamborn KR, Chang SM, et al. Volume of residual disease as a predictor of outcome in adult patients with recurrent supratentorial glioblastomas multiforme who are undergoing chemotherapy. J Neurosurg. 2004;100:41–46.
Barzon L, Boscaro M, Palù G . Endocrine aspects of cancer gene therapy. Endocr Rev. 2004;25:1–44.
Rainov NG . A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–2401.
van Dillen IJ, Mulder NH, Vaalburg W, et al. Influence of the bystander effect on HSV-tk/GCV gene therapy. A review. Curr Gene Ther. 2002;2:307–322.
Barba D, Hardin J, Sadelain M, Gage FH . Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci USA. 1994;91:4348–4352.
Kramm CM, Korholz D, Rainov NG, et al. Systemic activation of the immune system during ganciclovir treatment following intratumoral herpes simplex virus type 1 thymidine kinase gene transfer in an adolescent ependymoma patient. Neuropediatrics. 2002;33:6–9.
Rainov NG, Kramm CM, Banning U, et al. Immune response induced by retrovirus-mediated HSV-tk/GCV pharmacogene therapy in patients with glioblastoma multiforme. Gene Therapy. 2000;7:1853–1858.
Ram Z, Culver KW, Oshiro EM, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361.
Shand N, Weber F, Mariani L, et al. A phase 1–2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European–Canadian Study Group. Hum Gene Ther. 1999;10:2325–2335.
Pizzato M, Franchin E, Calvi P, et al. Production and characterization of a bicistronic Moloney-based retroviral vector expressing human interleukin 2 and herpes simplex virus thymidine kinase for gene therapy of cancer. Gene Therapy. 1998;5:1003–1007.
Barzon L, Bonaguro R, Castagliuolo I, et al. Transcriptionally targeted retroviral vector for combined suicide and immunomodulating gene therapy of thyroid cancer. J Clin Endocrinol Metab. 2002;87:5304–5311.
Barzon L, Bonaguro R, Castagliuolo I, et al. Gene therapy of thyroid cancer via retrovirally-driven combined expression of human interleukin-2 and herpes simplex virus thymidine kinase. Eur J Endocrinol. 2003;148:73–80.
Palù G, Cavaggioni A, Calvi P, et al. Gene therapy of glioblastoma multiforme via combined expression of suicide and cytokine genes: a pilot study in humans. Gene Therapy. 1999;6:330–337.
Oldfield EH, Ram Z, Culver KW, et al. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993;4:39–69.
Colombo F, Zanusso M, Casentini L, et al. Gene stereotactic neurosurgery for recurrent malignant gliomas. Stereotact Funct Neurosurg. 1997;68:245–251.
Rho HM, Poiesz B, Ruscetti FW, et al. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology. 1981;112:355–360.
Cosset F-L, Takeuchi Y, Battini J-L, et al. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436.
Macdonald DR, Terrance LC, Cascino S, et al. Response criteria for phase II studies of supratentorial malignant gliomas. J Clin Oncol. 1990;8:1277–1280.
Brandes AA, Scelzi E, Salmistraro G, et al. Incidence and risk of thromboembolism during treatment of high-grade gliomas: a prospective study. Eur J Cancer. 1997;33:1592–1596.
De Cicco M . The prothrombotic state in cancer: pathogenic mechanisms. Crit Rev Oncol Hematol. 2004;50:187–196.
Long Z, Li LP, Grooms T, et al. Biosafety monitoring of patients receiving intracerebral injections of murine retroviral vector producer cells. Hum Gene Ther. 1998;9:1165–1172.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419.
Kohn DB, Sadelain M, Dunbar C, et al. American Society of Gene Therapy (ASGT) ad hoc subcommittee on retroviral-mediated gene transfer to hematopoietic stem cells. Mol Ther. 2003;8:180–187.
Klatzmann D, Valery CA, Bensimon G, et al. A phase I/II study of herpes simplex virus type 1 thymidine kinase “suicide” gene therapy for recurrent glioblastoma. Study Group on Gene Therapy for Glioblastoma. Hum Gene Ther. 1998;9:2595–2604.
Izquierdo M, Martin V, Izquierdo JM, et al. Human malignant brain tumor response to herpes simplex thymidine kinase (HSVtk)/ganciclovir gene therapy. Gene Therapy. 1996;3:491–495.
Sobol RE, Fakhrai H, Shawler D, et al. Interleukin-2 gene therapy in a patient with glioblastoma. Gene Therapy. 1995;2:164–167.
Sampath P, Hanes J, DiMeco F, et al. Paracrine immunotherapy with interleukin-2 and local chemotherapy is synergistic in the treatment of experimental brain tumors. Cancer Res. 1999;59:2107–2114.
Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518.
Immonen A, Vapalahti M, Tyynela K, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomized, controlled study. Mol Ther. 2004;10:967–972.
Trask TW, Trask RP, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with recurrent malignant brain tumors. Mol Ther. 2000;1:195–203.
Sandmair AM, Loimas S, Puranen P, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197–2205.
Acknowledgements
This work was supported by grants from MIUR no. 2002062741 and FIRB no. RBNE0127YS-006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colombo, F., Barzon, L., Franchin, E. et al. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther 12, 835–848 (2005). https://doi.org/10.1038/sj.cgt.7700851
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700851
Keywords
This article is cited by
-
Recent progress in the research of suicide gene therapy for malignant glioma
Neurosurgical Review (2021)
-
Bicistronic transfer of CDKN2A and p53 culminates in collaborative killing of human lung cancer cells in vitro and in vivo
Gene Therapy (2020)
-
Quo Vadis—Do Immunotherapies Have a Role in Glioblastoma?
Current Treatment Options in Neurology (2018)
-
Dendritic cell immunotherapy for brain tumors
Journal of Neuro-Oncology (2015)
-
Marmosets as a preclinical model for testing “off-label” use of doxycycline to turn on Flt3L expression from high-capacity adenovirus vectors
Molecular Therapy - Methods & Clinical Development (2014)