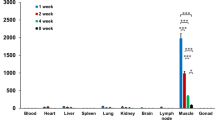
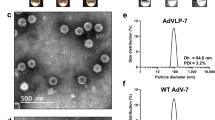
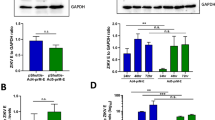
Abstract
Replication-competent (RC) adenoviruses (Ads) are increasingly being developed as oncolytic vectors and as vehicles for delivering vaccine antigens. Although the safety of such vectors in humans is of paramount importance, these vectors pose additional special concerns. Specifically, the prospect of causing Ad-mediated disease in the patient, the amount and sites of Ad replication, the possibility of virus shedding leading to unintended transmission to patient contacts, and the potential for persistence in the inoculated individual must be evaluated. Previous experience with administration of wild-type and RC recombinant Ads to humans may shed light on some of these issues. Experimental infections of humans with natural Ad isolates and RC recombinant vectors show that in adults Ads cause mild or no disease, particularly with Ad serotypes 2 and 5, the serotypes most often used to make recombinant constructs. Other studies show that Ad can replicate in experimentally infected persons, that in some situations Ads can be shed and transmitted to close contacts, and that there is evidence for persistent/latent Ad infection in naturally infected individuals. Overall, these studies indicate that Ads can be safely administered to humans for the treatment of cancer and as antigen delivery vehicles suggesting that the continued development of RC oncolytic and vaccine vectors should be pursued.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rowe WP, Huebner RJ, Gillmore LK, Parrott RH, Ward TG . Isolation of a cytopathic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953;84:570–573.
Hilleman MR, Werner JH . Recovery of new agents from patients with acute respiratory illness. Proc Soc Exp Biol Med. 1954;85:183–188.
Huebner RJ, Rowe WP, Ward TG, Parrott RH, Bell JA . Adenoidal—pharyngoconjunctival agents. N Engl J Med. 1954;251:1077–1086.
Horwitz MS . Adenoviruses. In: Knipe DM, Howley PM, eds. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001: 2301–2326.
Commission on Acute Respiratory Disease. Experimental transmission of minor respiratory illness to human volunteers by filter-passing agents. I. Demonstration of two types of illness characterized by long and short incubation periods and different clinical features. J Clin Invest. 1947;26:957–982.
Bell JA, Ward TG, Huebner RJ, Rowe WP, Suskind RG, Paffenbarger RS . Studies of adenoviruses (APC) in volunteers. Am J Public Health. 1956;46:1130–1146.
Chaproniere DM, Pereira HG, Roden AT . Infection of volunteers by a virus (A.P.C. type 1) isolated from human adenoid tissue. Lancet. 1956;271:592–596.
Ward TG, Huebner RJ, Rowe WP, Ryan RW, Bell JA . Production of pharyngoconjunctival fever in human volunteers inoculated with APC virus. Science. 1955;122:1086–1087.
Couch RB, Knight V, Douglas Jr RG, Black BH, Hamory SH . The minimal infectious dose of adenovirus type 4; the case for natural transmission by viral aerosol. Trans Am Clin Climatol Assoc. 1969;80:205–211.
Couch RB, Cate TR, Fleet WF, Gerone PJ, Knight V . Aerosol-induced adenoviral illness resembling the naturally occurring illness in military recruits. Am Rev Respir Dis. 1966;93:529–535.
Mitsui Y, Hanabusa J, Minoda R, Ogata S . Effect of inoculating adenovirus (APC virus) type 8 into human volunteers. Am J Ophthalmol. 1957;43:84–90.
Mitsui Y, Hanna L, Minoda R, et al. Experiments in human volunteers with adenovirus type 8. Br J Ophthalmol. 1959;43:540–547.
Jawetz E, Hanna L, Sonne M, Thygeson P . A laboratory infection with adenovirus type 8; laboratory and epidemiologic observations. Am J Hyg. 1959;69:13–20.
Kasel JA, LODA F, Knight V . Infection of volunteers with adenovirus type 16. Proc Soc Exp Biol Med. 1963;114:621–623.
Kasel JA, Evans HE, Spickard A, Knight V . Conjunctivitis and enteric infection with adenovirus types 26 and 27: responses to primary, secondary and reciprocal cross-challenges. Am J Hyg. 1963;77:265–282.
Chanock RM, Ludwig W, Huebner RJ, Cate TR, Chu LW . Immunization by selective infection with type 4 adenovirus grown in human diploid tissue cultures. I. Safety and lack of oncogenicity and tests for potency in volunteers. JAMA. 1966;195:445–452.
Edmondson WP, Purcell RH, Gundelfinger BF, Love JW, Ludwig W, Chanock RM . Immunization by selective infection with type 4 adenovirus grown in human diploid tissue culture. II. specific protective effect against epidemic disease. JAMA. 1966;195:453–459.
Top Jr FH, Buescher EL, Bancroft WH, Russell PK . Immunization with live types 7 and 4 adenovirus vaccines II Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. J Infect Dis. 1971;124:155–160.
Top Jr FH, Grossman RA, Bartelloni PJ, et al. Immunization with live types 7 and 4 adenovirus vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J Infect Dis. 1971;124:148–154.
van der Veen J, Abarbanel MFW, Oei KG . Vaccination with live type 4 adenovirus: evaluation of antibody response and protective efficacy. J Hyg. 1968;66:499–511.
Dudding BA, Bartelloni PJ, Scott RM, Top Jr FH, Russell PK, Buescher EL . Enteric immunization with live adenovirus type 21 vaccine. I. Tests for safety, infectivity, immunogenicity, and potency in volunteers. Infect Immun. 1972;5:295–299.
Scott RM, Dudding BA, Romano SV, Russell PK . Enteric immunization with live adenovirus type 21 vaccine. II. Systemic and local immune responses following immunization. Infect Immun. 1972;5:300–304.
Rosenbaum MJ, De Berry P, Sullivan EJ, et al. Characteristics of vaccine-induced and natural infection with adenovirus type 4 in naval recruits. Am J Epidemiol. 1968;88:45–54.
Couch RB, Chanock RM, Cate TR, Lang DJ, Knight V, Huebner RJ . Immunization with types 4 and 7 adenovirus by selective infection of the intestinal tract. Am Rev Respir Dis. 1963;88 (Suppl-394–403).
Gutekunst RR, White RJ, Edmondson WP, Chanock RM . Immunization with live type 4 adenovirus: determination of infectious virus dose and protective effect of enteric infection. Am J Epidemiol. 1967;86:341–349.
Gaydos CA, Gaydos JC . Adenovirus vaccines in the U.S. military. Mil Med. 1995;160:300–304.
Smith TJ, Buescher EL, Top Jr FH, Altemeier WA, McCown JM . Experimental respiratory infection with type 4 adenovirus vaccine in volunteers: clinical and immunological responses. J Infect Dis. 1970;122:239–248.
Stanley ED, Jackson GG . Spread of enteric live adenovirus type 4 vaccine in married couples. J Infect Dis. 1969;119:51–59.
Mueller RE, Muldoon RL, Jackson GG . Communicability of enteric live adenovirus type 4 vaccine in families. J Infect Dis. 1969;119:60–66.
Schwartz AR, Togo Y, Hornick RB . Clinical evaluation of live, oral types 1, 2, and 5 adenovirus vaccines. Am Rev Respir Dis. 1974;109:233–239.
Lubeck MD, Davis AR, Chengalvala M, et al. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci USA. 1989;86:6763–6767.
Bhat BM, Bhat RA, Chengalvala MV, et al. Comparative analysis of high expression adeno-HBsAg recombinants with and without E3 region proteins in tissue culture, dogs, and chimpanzees. In: Norrby E, Brown F, Chanock RM, Ginsberg HS, eds. Vaccines 94. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1994: 309–314.
Chengalvala MV, Bhat BM, Bhat RA, et al. Replication and immunogenicity of Ad7-, Ad4-, and Ad5-hepatitis B virus surface antigen recombinants, with or without a portion of E3 region, in chimpanzees. Vaccine. 1997;15:335–339.
Lichtenstein DL, Toth K, Doronin K, Tollefson AE, Wold WS . Functions and mechanisms of action of the adenovirus E3 proteins. Int Rev Immunol. 2004;23:75–111.
Tacket CO, Losonsky G, Lubeck MD, et al. Initial safety and immunogenicity studies of an oral recombinant adenohepatitis B vaccine. Vaccine. 1992;10:673–676.
Lubeck MD, Natuk RJ, Chengalvala M, et al. Immunogenicity of recombinant adenovirus-human immunodeficiency virus vaccines in chimpanzees following intranasal administration. AIDS Res Hum Retroviruses. 1994;10:1443–1449.
Lubeck MD, Natuk R, Myagkikh M, et al. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med. 1997;3:651–658.
Evans AS . Latent adenovirus infections of the human respiratory tract. Am J Hyg. 1958;67:256–266.
Israel MS . The viral flora of enlarged tonsils and adenoids. J Path Bacteriol. 1962;84:169–176.
Nász I, Tóth M, Lengyel A . Adenoviruses isolated from excised tonsils. Acta Microbiol Acad Sci Hung. 1958;5:267–269.
Schlesinger RW . Vagaries of adenovirus-cell complexes. In: Pollard M, ed. Perspectives in Virology. Minneapolis: Burgess; 1961: 69–77.
Strohl WA, Schlesinger RW . Quantitative studies of natural and experimental adenovirus infection of human cells. II. Primary cultures and the possible role of asynchronous viral multiplication in the maintenance of infection. Virology. 1965;26:208–220.
van der Veen J, Lambriex M . Relationship of adenovirus to lymphocytes in naturally infected human tonsils and adenoids. Infect Immun. 1973;7:604–609.
Zaiman E, Balducci D, Tyrrell DA . A.P.C. viruses and respiratory disease in Northern England. Lancet. 1955;269:595–596.
Neumann R, Genersch E, Eggers HJ . Detection of adenovirus nucleic acid sequences in human tonsils in the absence of infectious virus. Virus Res. 1987;7:93–97.
Fox JP, Brandt CD, Wassermann FE, et al. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families VI Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969;89:25–50.
Fox JP, Hall CE, Cooney MK . The Seattle Virus Watch. VII. Observations of adenovirus infections. Am J Epidemiol. 1977;105:362–386.
Green M, Wold WSM, Mackey JK, Rigden P . Analysis of human tonsil and cancer DNAs and RNAs for DNA sequences of group C (serotypes 1, 2, 5, and 6) human adenoviruses. Proc Natl Acad Sci USA. 1979;76:6606–6610.
Horvath J, Palkonyay L, Weber J . Group C adenovirus DNA sequences in human lymphoid cells. J Virol. 1986;59:189–192.
Jones KW, Kinross J, Maitland N, Norval M . Normal human tissues contain RNA and antigens related to infectious adenovirus type 2. Nature. 1979;277:274–279.
Matsuse T, Hayashi S, Kuwano K, Keunecke H, Jefferies WA, Hogg JC . Latent adenoviral infection in the pathogenesis of chronic airways obstruction. Am Rev Respir Dis. 1992;146:177–184.
Elliott WM, Hayashi S, Hogg JC . Immunodetection of adenoviral E1A proteins in human lung tissue. Am J Respir Cell Mol Biol. 1995;12:642–648.
Retamales I, Elliott WM, Meshi B, et al. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164:469–473.
Bateman ED, Hayashi S, Kuwano K, Wilke TA, Hogg JC . Latent adenoviral infection in follicular bronchiectasis. Am J Respir Crit Care Med. 1995;151:170–176.
Macek V, Sorli J, Kopriva S, Marin J . Persistent adenoviral infection and chronic airway obstruction in children. Am J Respir Crit Care Med. 1994;150:7–10.
Macek V, Dakhama A, Hogg JC, Green FH, Rubin BK, Hegele RG . PCR detection of viral nucleic acid in fatal asthma: is the lower respiratory tract a reservoir for common viruses? Can Respir J. 1999;6:37–43.
Marin J, Jeler-Kacar D, Levstek V, Macek V . Persistence of viruses in upper respiratory tract of children with asthma. J Infect. 2000;41:69–72.
Eissa NT, Chu CS, Danel C, Crystal RG . Evaluation of the respiratory epithelium of normals and individuals with cystic fibrosis for the presence of adenovirus E1a sequences relevant to the use of E1a- adenovirus vectors for gene therapy for the respiratory manifestations of cystic fibrosis. Hum Gene Ther. 1994;5:1105–1114.
Kuwano K, Kawasaki M, Kunitake R, et al. Detection of group C adenovirus DNA in small-cell lung cancer with the nested polymerase chain reaction. J Cancer Res Clin Oncol. 1997;123:377–382.
Kuwano K, Nomoto Y, Kunitake R, et al. Detection of adenovirus E1A DNA in pulmonary fibrosis using nested polymerase chain reaction. Eur Respir J. 1997;10:1445–1449.
Flomenberg P, Piaskowski V, Harb J, Segura A, Casper JT . Spontaneous, persistent infection of a B-cell lymphoma with adenovirus. J Med Virol. 1996;48:267–272.
Flomenberg P, Gutierrez E, Piaskowski V, Casper JT . Detection of adenovirus DNA in peripheral blood mononuclear cells by polymerase chain reaction assay. J Med Virol. 1997;51:182–188.
Garnett CT, Erdman D, Xu W, Gooding LR . Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002;76:10608–10616.
Smith RR, Huebner RJ, Rowe WP, Schatten WE, Thomas LB . Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9:1211–1218.
Southam CM, Hilleman MR, Werner JH . Pathogenicity and oncolytic capacity of RI virus strain RI-67 in man. J Lab Clin Med. 1956;47:573–582.
Georgiades J, Zielinski T, Cicholska A, Jordan E . Research on the oncolytic effect of APC viruses in cancer of the cervix uteri; preliminary report. Biul Inst Med Morsk Gdansk. 1959;10:49–57.
Zielinski T, Jordan E . [Remote results of clinical observation of the oncolytic action of adenoviruses on cervix cancer]. Nowotwory. 1969;19:217–221.
Shenk T . Adenoviridae: the viruses and their replication. In: Knipe DM, Howley PM, eds. Fields Virology. Philadelphia: Lippincott, Williams & Wilkins; 2001: 2265–2300.
Balmain A, Gray J, Ponder B . The genetics and genomics of cancer. Nat Genet. 2003;33 (Suppl):238–244.
Coultas L, Strasser A . The role of the Bcl-2 protein family in cancer. Semin Cancer Biol. 2003;13:115–123.
Oren M . Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442.
Sherr CJ . Principles of tumor suppression. Cell. 2004; 116:235–246.
Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376.
Khuri FR, Nemunaitis J, Ganly I, et al. A controlled trial of intratumoral ONYX-015, a selectively replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885.
Nemunaitis J, Cunningham C, Buchanan A, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Therapy. 2001;8:746–759.
Reid T, Galanis E, Abbruzzese J, et al. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Therapy. 2001;8:1618–1626.
Lamont JP, Nemunaitis J, Kuhn JA, Landers SA, McCarty TM . A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience). Ann Surg Oncol. 2000;7:588–592.
Nemunaitis J, Khuri F, Ganly I, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298.
Habib NA, Sarraf CE, Mitry RR, et al. E1B-deleted adenovirus (dl1520) gene therapy for patients with primary and secondary liver tumors. Hum Gene Ther. 2001;12:219–226.
Hecht JR, Bedford R, Abbruzzese JL, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561.
Hamid O, Varterasian ML, Wadler S, et al. Phase II trial of intravenous CI-1042 in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:1498–1504.
Makower D, Rozenblit A, Kaufman H, et al. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin Cancer Res. 2003;9:693–702.
Reid T, Galanis E, Abbruzzese J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079.
Rudin CM, Cohen EE, Papadimitrakopoulou VA, et al. An attenuated adenovirus, ONYX-015, as mouthwash therapy for premalignant oral dysplasia. J Clin Oncol. 2003;21:4546–4552.
Vasey PA, Shulman LN, Campos S, et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol. 2002;20:1562–1569.
Habib N, Salama H, Abd El Latif Abu Median, et al. Clinical trial of E1B-deleted adenovirus (dl1520) gene therapy for hepatocellular carcinoma. Cancer Gene Ther. 2002;9:254–259.
Ganly I, Kirn D, Eckhardt G, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806.
Mulvihill S, Warren R, Venook A, et al. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Therapy. 2001;8:308–315.
Nemunaitis J, Ganly I, Khuri F, et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55 kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366.
Nemunaitis J, Cunningham C, Tong AW, et al. Pilot trial of intravenous infusion of a replication-selective adenovirus (ONYX-015) in combination with chemotherapy or IL-2 treatment in refractory cancer patients. Cancer Gene Ther. 2003;10:341–352.
Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR . Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–2563.
DeWeese TL, van der Poel H, Li S, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472.
Yu D-C, Chen Y, Seng M, Dilley J, Henderson DR . The addition of adenovirus type 5 region E3 enables Calydon virus 787 to eliminate distant prostate tumor xenografts. Cancer Res. 1999;59:4200–4203.
DeWeese T, Arterbery E, Michalski J, et al. A Phase I/II dose escalation trial of the intra prostatic injection of CG7870, a prostate specific antigen-dependent oncolytic adenovirus in patients with locally recurrent prostate cancer following definitive radiotherapy. Cancer Gene Ther. 2003;10:S13–S14.
Freytag SO, Khil M, Stricker H, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002;62:4968–4976.
Freytag SO, Stricker H, Pegg J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506.
Freytag SO, Rogulski KR, Paielli DL, Gilbert JD, Kim JH . A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9:1323–1333.
Benjamin R, Helman L, Meyers P, Reaman G . A phase I/II dose escalation and activity study of intravenous injections of OCaP1 for subjects with refractory osteosarcoma metastatic to lung. Hum Gene Ther. 2001;12:1591–1593.
Matsubara S, Wada Y, Gardner TA, et al. A conditional replication-competent adenoviral vector, Ad-OC-E1a, to cotarget prostate cancer and bone stroma in an experimental model of androgen-independent prostate cancer bone metastasis. Cancer Res. 2001;61:6012–6019.
Anon. Assessment of adenoviral vector safety and toxicity: report of the National Institutes of Health Recombinant DNA Advisory Committee. Hum Gene Ther. 2002;13:3–13.
Heise CC, Williams AM, Xue S, Propst M, Kirn DH . Intravenous administration of ONYX-015, a selectively replicating adenovirus, induces antitumoral efficacy. Cancer Res. 1999;59:2623–2628.
Lieber A, He CY, Meuse L, et al. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807.
Muruve DA, Barnes MJ, Stillman IE, Libermann TA . Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976.
Yver A . Does detection of circulating ONYX-015 genome by polymerase chain reaction indicate vector replication? J Clin Oncol. 2001;19:3155–3156.
Nemunaitis J, Cunningham C . Does detection of circulating ONYX-015 genome by polymerase chain reaction indicate vector replication? J Clin Oncol. 2001;19:3156–3157.
Peters AH, Drumm J, Ferrell C, et al. Absence of germline infection in male mice following intraventricular injection of adenovirus. Mol Ther. 2001;4:603–613.
Ye X, Gao GP, Pabin C, Raper SE, Wilson JM . Evaluating the potential of germ line transmission after intravenous administration of recombinant adenovirus in the C3H mouse. Hum Gene Ther. 1998;9:2135–2142.
Paielli DL, Wing MS, Rogulski KR, et al. Evaluation of the biodistribution, persistence, toxicity, and potential of germ-line transmission of a replication-competent human adenovirus following intraprostatic administration in the mouse. Mol Ther. 2000;1:263–274.
Acknowledgements
We thank Dr Dorota Skowyra for the Polish to English translation of reference number 67. This work was supported by Grants CA081829, CA108335, CA108046, and CA108046 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lichtenstein, D., Wold, W. Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: replication, safety, and transmission. Cancer Gene Ther 11, 819–829 (2004). https://doi.org/10.1038/sj.cgt.7700765
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700765
Keywords
This article is cited by
-
Icosahedral gold nanoparticles decorated with hexon protein: a surrogate for adenovirus serotype 5
Analytical and Bioanalytical Chemistry (2023)
-
A fully replication-competent adenovirus vector with enhanced oncolytic properties
Cancer Gene Therapy (2010)
-
A Phase I Study of Telomerase-specific Replication Competent Oncolytic Adenovirus (Telomelysin) for Various Solid Tumors
Molecular Therapy (2010)
-
Environmental risk assessment for medicinal products containing genetically modified organisms
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2010)
-
INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies
Cancer Gene Therapy (2009)