Abstract
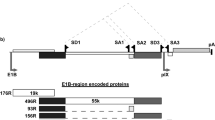
Replication-competent adenoviruses could provide an efficient method for delivering therapeutic genes to tumors. The most promising strategies among adenovirus-based oncolytic systems are designed to exploit free E2F-1 activity in cancer cells, which in the absence of pRb activates transcription and regulates the expression of genes involved in differentiation, proliferation, and apoptosis. We previously developed Δ24, an E1A-mutant, conditionally replicative oncolytic adenovirus. Here, we examine the ability of a second-generation Δ24 (Δ24-hyCD) engineered to express a humanized form of the Saccharomyces cerevisiae cytosine deaminase gene (hyCD). Real-time quantitative PCR, Western blotting, thin-layer chromatography, and radioisotope quantitative enzymatic assays confirmed the production of a catalytically active hyCD enzyme in the setting of an oncolytic infection in vitro; other experiments assessing local production of 5-fluorouracil and a concomitant bystander effect showed improved cytotoxicity. The IC50 dose of 5-fluorocytosine (5-FC) required for a complete cytopathic effect by the Δ24-hyCD virus was fivefold lower than with Δ24 alone in U251MG and U87MG malignant glioma (MG) cell lines. Intratumoral treatment of mice bearing intracranial U87MG xenografts with Δ24-hyCD+5-FC significantly improved survival, confirming that Δ24-hyCD with 5-FC is a more efficient anticancer tool than Δ24 alone. Histopathologically, Δ24-hyCD replication was accompanied by progressively augmented oncolysis and drug-induced necrosis. These findings demonstrate that Δ24-hyCD with concomitant systemic 5-FC is a significant improvement over the earlier Δ24 oncolytic tumor-selective strategy for therapy of experimental gliomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Central Brain Tumor Registry of the United States. Statistical Report: Primary Brain tumors in the United States, 1992–1997. (2001) Available at http://www.cbtrus.org/reports.htm (accessed November 21, 2002).
Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12.
Alemany R, Gomez-Manzano C, Balague C, et al. Gene therapy for gliomas: molecular targets, adenoviral vectors, and oncolytic adenoviruses. Exp Cell Res. 1999;252:1–12.
Henson JW, Schnitker BL, Correa KM, et al. The retinoblastoma gene is involved in malignant progression of astrocytomas. Ann Neurol. 1994;36:714–721.
Huang HJ, Yee JK, Shew JY, et al. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988;242:1563–1566.
Aghi M, Chou TC, Suling K, et al. Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/rat cytochrome P450 2B1 and ganciclovir/herpes simplex virus thymidine kinase gene therapies. Cancer Res. 1999;59:3861–3865.
Chase M, Chung RY, Chiocca EA . An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat Biotechnol. 1998;16: 444–448.
Boviatsis EJ, Park JS, Sena-Esteves M, et al. Long-term survival of rats harboring brain neoplasms treated with ganciclovir and a herpes simplex virus vector that retains an intact thymidine kinase gene. Cancer Res. 1994;54:5745–5751.
Hermiston T . Gene delivery from replication-selective viruses: arming guided missiles in the war against cancer. J Clin Invest. 2000;105:1169–1172.
Wildner O, Blaese RM, Morris JC, et al. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410–413.
Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376.
Freytag SO, Stricker H, Pegg J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506.
Rogulski K, Wing MS, Paielli DL, et al. Double suicide gene therapy augments the antitumor activity of a replication-competent lytic adenovirus through enhanced cytotoxicity and radiosensitization. Hum Gene Ther. 2000;11:67–76.
Bernt KM, Steinwaerder DS, Ni S, et al. Enzyme-activated prodrug therapy enhances tumor-specific replication of adenovirus vectors. Cancer Res. 2002;62:6089–6098.
Ueda K, Iwahashi M, Nakamori M, et al. Carcinoembryonic antigen-specific suicide gene therapy of cytosine deaminase/5-fluorocytosine enhanced by the Cre/loxP system in the orthotopic gastric carcinoma model. Cancer Res. 2001;61:6158–6161.
Miller CR, Williams CR, Buchsbaum DJ, et al. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002;62:773–780.
Erbs P, Exinger F, Jund R . Characterization of the Saccharomyces cerevisiae FCY1 gene encoding cytosine deaminase and its homologue FCA1 of Candida albicans. Curr Genet. 1997;31:1–6.
Hamstra DA, Rice DJ, Fahmy S, et al. Enzyme/prodrug therapy for head and neck cancer using a catalytically superior cytosine deaminase. Hum Gene Ther. 1999;10:1993–2003.
Whyte P, Williamson NM, Harlow E, et al. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989;56:67–75.
Blanquicett C, Gillespie GY, Nabors LB, et al. Induction of thymidine phosphorylase in both irradiated and shielded, contralateral human U87MG glioma xenografts: Implications for a dual modality treatment using capecitabine and irradiation. Mol Cancer Ther. 2002;1:1139–1145.
Rubery ED, Newton AA . A simple paper chromatographic method for separation of methylated adenines and cytosine from the major bases found in nucleic acids. Anal Biochem. 1971;42:149–154.
Lal S, Lacroix M, Tofilon P, et al. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92:326–333.
Kievit E, Bershad E, Ng E, et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59:1417–1421.
Roth JA, Cristiano RJ . Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89:21–39.
Ueki K, Ono Y, Henson JW, et al. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996;56:150–153.
Suzuki K, Fueyo J, Krasnykh V, et al. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001;7:120–126.
Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660.
Acknowledgements
We wish to thank Joann Aaron (Department of Neuro-Oncology) and Christine Wogan (Department of Scientific Publications) at MD Anderson Cancer Center for editorial services. We also acknowledge the technical assistance of the animal core facility staff at the MD Anderson Brain Tumor Center. This work was supported in part by grants from the Anthony Bullock III Foundation, the Jonsson Family Foundation and the Golfers Against Cancer organization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Conrad, C., Miller, C., Ji, Y. et al. Δ24-hyCD adenovirus suppresses glioma growth in vivo by combining oncolysis and chemosensitization. Cancer Gene Ther 12, 284–294 (2005). https://doi.org/10.1038/sj.cgt.7700750
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700750
Keywords
This article is cited by
-
Application of conditionally replicating adenoviruses in tumor early diagnosis technology, gene-radiation therapy and chemotherapy
Applied Microbiology and Biotechnology (2016)
-
VB-111: a novel anti-vascular therapeutic for glioblastoma multiforme
Journal of Neuro-Oncology (2015)
-
Chemovirotherapy of Malignant Melanoma with a Targeted and Armed Oncolytic Measles Virus
Journal of Investigative Dermatology (2013)
-
Oncolytic Herpes simplex virus expressing yeast cytosine deaminase: relationship between viral replication, transgene expression, prodrug bioactivation
Cancer Gene Therapy (2012)
-
The oncolytic adenovirus AdΔΔ enhances selective cancer cell killing in combination with DNA-damaging drugs in pancreatic cancer models
Gene Therapy (2011)