Abstract
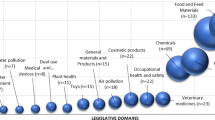
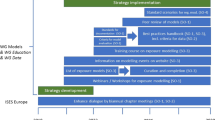
Under the new REACH system, companies importing, producing and marketing chemical substances will be obliged to register the single substances and to carry out a safety assessment for all identified uses during the life cycle of the substance. This duty will apply to about 10,000 existing substances in the EU market exceeding an annual production or import volume of 10 t per company. If the substance is already known to be dangerous or turns out to be dangerous1 during the hazard assessment, the registrant is obliged to carry out an exposure assessment and a risk characterisation for all identified uses. The goal of the safety assessment is to define the conditions of use that allow for adequate control of risk with regard to health and safety at the work place, consumer safety and protection of the environment. Once the registrant has established and documented these conditions in the Chemicals Safety Report (CSR), that information is to be communicated down the supply chain by means of the Extended Safety Data Sheet (eSDS). The ultimate aim of the new legislation is to establish duties and mechanisms that systematically prevent or limit exposure to dangerous industrial chemicals. The current paper explains this concept with regard to environmental exposure and highlights the challenges and possible solutions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Notes
“Article” means an object that during production is given a special shape, surface or design, which determines its function to a greater degree than does its chemical composition.
An initial exposure scenario is built on readily available information, and reflects the conditions of use as existing in the market. It should contain sufficient information to carry out a quantitative exposure assessment. A initial exposure scenario will be converted into a final exposure scenario during the iterative process of chemicals safety assessment.
Source: Online PBT information system http://ecb.jrc.it/esis/
In the current EU Technical Guidance Document on Risk Assessment (ECB, 2003), the fraction of main source is a default assumption in which fraction of the total market volume of a substance may be used and emitted at a single site at a maximum. The fraction of main source differs from sector to sector of use.
For example, CONEXPO, ECETOC Targeted Risk Assessment, or migration models applied for the assessment of food packaging.
A substance is dangerous when it meets the criteria for classification as dangerous according to EU-Directive 67/548/EEC or is assessed to be a persistent, bio-accumulative and toxic substances (PBT) or to be very persistent and very bio-accumulative (vPvB).
References
ECB. Technical Guidance Document on Risk Assessment, 2003 European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau.
Consortium RIP 3.2., 2006 Start-up-Guidance based on the preliminary Concise Technical Guidance Document (RIP 3.2.-2, August 2006), unpublished.
Preliminary Technical Guidance Document on preparing the Chemical Safety Assessment under REACH-Part B; Concise Preliminary Technical Guidance Document, p.73 (REACH Implementation Project 3.2-1B). Service contract number 22552-2004-12 F1SC ISP BE, http://ecb.irc.it/reach/rip.
REGULATION (EC) No 1907//2006 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), 18 December 2006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahrens, A., Traas, T. Environmental exposure scenarios: development, challenges and possible solutions. J Expo Sci Environ Epidemiol 17 (Suppl 1), S7–S15 (2007). https://doi.org/10.1038/sj.jes.7500602
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jes.7500602
Keywords
This article is cited by
-
Modeling bioconcentration factor (BCF) using mechanistically interpretable descriptors computed from open source tool ?PaDEL-Descriptor?
Environmental Science and Pollution Research (2014)