Abstract
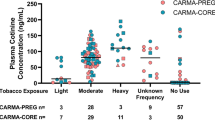
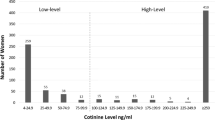
Environmental tobacco smoke (ETS) exposure has been studied in relation to many diseases. The ability of a study to find an association between exposure and disease is, in part, determined by the accuracy of the exposure measure. This study examined how accurately one question, the number of smokers in the household, asked at birth, predicts ETS exposure in pregnant nonsmokers as assessed by serum cotinine. Blood specimens, drawn at 15–19 weeks gestation, from 783 women who participated in a prenatal screening program in California in 1992 were analyzed for cotinine. Serum cotinine was significantly correlated with the number of smokers in the household (r=0.35, P<0.001, geometric mean cotinine (nanograms per milliliter) for 0 smokers=0.06, 1 smoker=0.18, 2 or more smokers=0.29). Using multiple regression, the number of smokers in the household accounted for 11% of the variation in serum cotinine. Cotinine concentrations were twice as high in women living with one or more smokers compared to women not living with a smoker, when reported exposure (0 or >0h) at home, work and other places was similar. Thus, the number of household smokers can account for a statistically significant amount of variation in serum cotinine and omission of this information would result in an underestimation of ETS exposure. Although use of this question alone does not provide an adequate estimation of ETS exposure as determined by serum cotinine, the results of this study indicate that this question is an important component of assessing ETS exposure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ahluwalia IB Brummer-Strawn L Scanion KS Exposure to environmental tobacco smoke and birth outcome: increased effects on pregnant women aged 30 years or older, Am J Epidemiol (1997) 146: 42–47
Becher H Zantonski W Jockel K-H Passive smoking in Germany and Poland: comparison of exposure levels, sources of exposure, validity, and perception, Epidemiology (1992) 3: 509–514
Benowitz NL Cotinine as a biomarker of environmental tobacco smoke exposure, Epidemiol Rev (1996) 18(2): 188–204
Benowitz NL Biomarkers of environmental tobacco smoke exposure, Environ Health Perspect (1999) 107(Suppl 2): 349–355
Benowitz NL Jacob P III Metabolism of nicotine to cotinine studies by a dual stable isotope method, Clin Pharmacol Ther (1994) 56: 483–493
Bernert JT Turner WE Pirkle JL Sosnoff CS Akins JR Waldrep MK Ann Q Covey TR Whitfield WE Gunter EW Miller BB Patterson DG Needham LL Hannon WH Sampson EJ Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry, Clin Chem (1997) 43(12): 2281–2291
Box GEP Cox DR An analysis of transformations, J R Stat Soc (1964) B-26: 211–243 discussion 244–252
Brooke OG Anderson HR Bland JM Peacock JL Stewart CM Effects on birthweight of smoking, alcohol, caffeine, socioeconomic factors and psychosocial stress, Br Med J (1989) 298(6676): 796–801
Chen Y Pederson LL Lefcoe NM Passive smoking and low birth weight [letter] Lancet 2 (1989) 8653: 54–55
Coghlin J Hammond SK Gann PH Development of epidemiologic tools for measuring environmental tobacco smoke exposure, Am J Epidemiol (1989) 130(4): 696–704
Coultas DB Howard CA Peake GT Skipper BJ Samet JM Discrepancies between self-reported and validated cigarette smoking in a community survey of New Mexico Hispanics, Am Rev Respir Dis (1988) 137: 810–814
Coultas DB Peake GT Samet JM Questionnaire assessment of lifetime and recent exposure to environmental tobacco smoke, Am J Epidemiol (1989) 130(2): 338–347
Cummings KM Markello SJ Mahoney M Bhargava AK McElroy PD Marshall JR Measurement of current exposure to environmental tobacco smoke, Arch Environ Health (1990) 45(2): 74–79
Dejin-Karlsson E Hanson BS Ostergren PO Sjoberg N-O Marsal K Does passive smoking in early pregnancy increase the risk of small-for-gestational-age infants?, Am J Public Health (1998) 88(10): 1523–1527
DeLorenze GN Kharrazi M Kaufman FL Eskenazi B Bernert JT . Environ Res (2002) (in press)
Emmons KM Abrams DB Marshall R Marcus BH Kane M Novotny TE Etzel RA An evaluation of the relationship between self-report and biochemical measures of environmental tobacco smoke exposure, Prev Med (1994) 23(1): 35–39
Eskenazi B Prehn AW Christianson RE Passive and active maternal smoking as measured by serum cotinine: the effect on birthweight, Am J Public Health (1995) 85: 395–398
Fortier I Marcoux S Brisson J Passive smoking during pregnancy and the risk of delivering a small-for-gestational-age infant, Am J Epidemiol (1994) 139(3): 294–301
Glantz SA Parmley WW Passive smoking and heart disease. Mechanisms and risk, JAMA (1995) 273: 1047–1053
Haddow JE Knight GJ Palomaki GE Kloza EM Wald NJ Cigarette consumption and serum cotinine in relation to birthweight, Br J Obstet Gynaecol (1987) 94: 678–681
Haddow JE Knight GJ Palomaki GE McCarthy JE Second-trimester serum cotinine levels in nonsmokers in relation to birth weight, Am J Obstet Gynecol (1988) 159(2): 481–484
Haley NJ Colosimo SG Axelrad CM Harris R Sepkovic DW Biochemical validation of self-reported exposure to environmental tobacco smoke, Environ Res (1989) 49: 127–135
Henschen M Frischer T Pracht T Spiekerkotter E Karmaus W Meinert R Lehnert W Wehrle E Kuehr J The internal dose of passive smoking at home depends on the size of the dwelling, Environ Res (1997) 72(1): 65–71
Jaro M Probabilistic linkage of large public health data files, Stat Med (1995) 14: 491–498
Jarvis MJ Tunstall-Pedoe H Feyerabend C Vesey C Saloojee Y Comparison of tests used to distinguish smokers from nonsmokers, Am J Public Health (1987) 77(11): 1435–1438
Kemmeren JM van Poppel G Verhoef P Jarvis MJ Plasma cotinine: stability in smokers and validation of self-reported smoke exposure in nonsmokers, Environ Res (1994) 66(2): 235–243
Lambers DS Clark KE The maternal and fetal physiologic effects of nicotine, Semin Perinatol (1996) 20(2): 115–126
Mainous AG III Hueston WJ Passive smoke and low birth weight, Arch Fam Med (1994) 3: 875–878
Martin TR Bracken MB Association of low birth weight with passive smoke exposure in pregnancy, Am J Epidemiol (1986) 124: 633–642
Martinez FD Wright AL Taussig LM The effect of paternal smoking on the birthweight of newborns whose mothers did not smoke, Am J Public Health (1994) 84(9): 1489–1491
Mathai M Vijayasri R Babu S Jeyaseelan L Passive maternal smoking and birthweight in a South Indian population, Br J Obstet Gynaecol (1992) 99: 342–343
Nelson PR deBethizy JD Davis RA Oldaker GB III Where there's smoke…? Biases in the use of nicotine and cotinine as environmental tobacco smoke biomarkers In Proceedings of the 1991 EPA/A&WMA International Symposium: Measurement of Toxic and Related Air Pollutants. Vol. 1 Air and Waste Management Association, Pittsburgh, PA 1991 449–454
O'Connor TZ Holfor TR Leaderer BP Hammond SK Bracken MB Measurement of exposure to environmental tobacco smoke in pregnant women, Am J Epidemiol (1995) 142: 1315–1321
Office of Environmental Health Hazard Assessment (OEHHA) . Health Effects of Exposure to Environmental Tobacco Smoke. California Environmental Protection Agency, Sacramento, CA 1997
Peacock JL Cook DG Carey IM Jarvis MJ Bryant AE Anderson HR Bland JM Maternal cotinine level during pregnancy and birthweight for gestational age, Int J Epidemiol (1988) 27(4): 647–656
Perez-Stable EJ Herrera B Jacob P III Benowitz NL Nicotine metabolism and intake in black and white smokers, JAMA (1998) 280(2): 152–156
Pirkle JL Flegal KM Bernert JT Brody DJ Etzel RA Maurer KR Exposure of the US population to environmental tobacco smoke, JAMA (1996) 275(16): 1233–1240
Pregibon D Data Analytic Methods for Generalized Linear Models PhD dissertation University of Toronto 1979
Pregibon D Goodness of link tests for generalized linear models, Appl Stat (1980) 29: 15–24
Rebagliato M Florey C Bolumar F Exposure to environmental tobacco smoke in nonsmoking pregnant women in relation to birth weight, Am J Epidemiol (1995a) 142: 531–537
Rebagliato M Bolumar F Florey C Assessment of exposure to environmental tobacco smoke in nonsmoking pregnant women in different environments of daily living, Am J Epidemiol (1995b) 142: 525–530
Riboli E Preston-Martin S Saracci R Haley NJ Trichopoulos D Becher H Burch JD Fontham ET Gao YT Jindal SK Koo LC Marchand LL Segnan N Shimizu H Stanta G Wu-Williams AH Zatonski W Exposure of nonsmoking women to environmental tobacco smoke: a 10-country collaborative study, Cancer Causes Control (1990) 1: 243–252
Roquer JM Figueras J Botet F Jimenez R Influence on fetal growth of exposure to tobacco smoke during pregnancy, Acta Paediatr (1994) 84: 118–121
Saito R The smoking habits of pregnant women and their husbands, and the effect on their infants, Nippon Koshu Eisei Zasshi (1991) 38: 124–131
Spitzer WO Lawrence V Dales R Hill G Archer MC Clark P Abenhaim L Hardy J Sampalis J Pinfold SP Morgan PP Links between passive smoking and disease: a best-evidence synthesis. A report of the working group on passive smoking, Clin Invest Med (1990) 13: 17–42
Taylor AE Johnson DC Kazemi H Environmental tobacco smoke and cardiovascular disease. A position paper from the council on cardiopulmonary and critical care, American Heart Association, Circulation (1992) 86: 699–702
Taylor JK Principles of measurement In Quality Assurance of Chemical Measurements. Lewis Publishers, Chelsea, MI 1987 75–93
Tukey JW One degree of freedom for non-additivity, Biometrics (1949) 5: 232–242
U.S. Department of Health and Human Services (USDHHS) The health consequences of smoking for women: a report of the Surgeon General US DHHS publication no. 410-889/12840 U.S. Department of Health and Human Services, Rockville, MD 1980
U.S. Department of Health and Human Services (USDHHS). Women and smoking: a Report of the Surgeon General. USDHHS, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health: Atlanta, GA 2001
U.S. Environmental Protection Agency (US EPA) Respiratory health effects of passive smoking: lung cancer and other disorders EPA publication no. 600/6-90/006F Off. Health Environ. Assess., US EPA, Washington, DC 1992
Zhang J Ratcliffe JM Paternal smoking and birthweight in Shanghai, Am J Public Health (1993) 83(2): 207–210
Acknowledgements
This research was supported by the California Tobacco-Related Disease Research Program (Grant No. 6RT-0385). The data collection was aided by March of Dimes Birth Defects Foundation Grant Nos. 15-FY92-0078 and 15-FY93-0662. The authors thank Lynn Goldman, George Cummingham, Robert Haas, Richard Kreutzer, Enid Satariano, Steve Graham, Cindy Evangelista, and Betsy Noth.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
KAUFMAN, F., KHARRAZI, M., DELORENZE, G. et al. Estimation of environmental tobacco smoke exposure during pregnancy using a single question on household smokers versus serum cotinine. J Expo Sci Environ Epidemiol 12, 286–295 (2002). https://doi.org/10.1038/sj.jea.7500224
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jea.7500224
Keywords
This article is cited by
-
Prevalence and risk factors of secondhand smoke (SHS) exposure among pregnant women in Mongolia
Scientific Reports (2017)
-
Kunnen kinderen symptomen van nicotineafhankelijkheid ontwikkelen door blootstelling aan omgevingsrook?
Kind en adolescent (2016)
-
Identification and comparison of chromosomal alterations in infertile and fertile males of Tamil Nadu region exposed to cigarette smoking
Journal of Public Health (2011)
-
A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: the correlation between serum and meconium and their association with infant birth weight
Environmental Health (2010)
-
Fetal Exposure to Secondhand Tobacco Smoke Assessed by Maternal Self-reports and Cord Blood Cotinine: Prospective Cohort Study in Krakow
Maternal and Child Health Journal (2009)