ABSTRACT
The dynamic distribution of phosphorylated Histone H3 on Ser10 (phospho-H3) in cells was investigated to determine its function during mitosis. Human breast adenocarcinoma cells MCF-7, and Chinese hamster cells CHO were analyzed by indirect immunofluorescence staining with an antibody against phospho-H3. We found that the phosphorylation begins at early prophase, and spreads throughout the chromosomes at late prophase. At metaphase, most of the phospho-H3 aggregates at the end of the condensed entity of chromosomes at equatorial plate. During anaphase and telophase, the fluorescent signal of phospho-H3 is detached from chromosomes into cytoplasm. At early anaphase, phospho-H3 shows ladder bands between two sets of separated chromosome, and forms “sandwich-like structure” when the chromosomes condensed. With the cleavage progressing, the “ladders” of the histone contract into a bigger bright dot. Then the histone aggregates and some of compacted microtubules in the midbody region are composed into a “bar-like” complex to separate daughter cells. The daughter cells seal their plasma membrane along with the ends of the “bar”, inside which locates microtubules and modified histones, to finish the cytokinesis and keep the “bar complex” out of the cells. The specific distribution and kinetics of phospho-H3 in cytoplasm suggest that the modified histones may take part in the formation of midbody and play a crucial role in cytokinesis.
Similar content being viewed by others
INTRODUCTION
In eukaryotic cells, DNAs bind to alkaline proteins histones to constitute a nucleosome. Histones are subject to a variety of post-translation modifications, including acetylation and methylation of lysines and arginines, phosphorylation of serines and threonines, as well as ubiquitylation and ribosylation. Particular patterns of histone modifications correlate with global chromatin dynamics, for instance, phosphorylation of histone H2A and H3 appears to be a hallmark of condensed mitotic chromatin 1, 2, 3, 4.
It was previously demonstrated that phosphorylation of linker histones, H1, is not necessarily required for mitotic or meiotic chromosome condensation 5, 6. However, recent studies have shown that phosphorylation of the amino-terminal domain of H3 (S10) is spatially and temporally related to chromosome condensation 7, 8, 9. The relationship between chromosome condensation and histone H3 phosphorylation was verified by electronic microscopy and high resolution of gel electrophoresis. Using the antibody specific to phospho-H3 and immunofluorescence labeling, Hendrel et al extended previous biochemical studies by demonstrating that mitotic phosphorylation of H3 initiated in pericentromeric heterochromatin in late G2 interphase cell, spreaded throughout the condensing chromatin, and completed in most mammalian cells just prior to entry metaphase. Dephosphorylation of H3 began in anaphase and ceased immediately prior to detectable chromosome decondensation in telophase 7. Wei et al demonstrated that micronuclear H3 phosphorylation occurred at a single site (Ser10) in the amino-terminal domain of histone H3 in ciliated protozoan and correlated with mitotic and meiotic division of micronuclei in a pattern that closely coincide with chromosome condensation 10. Genetic studies in Tetrahymena showed that a mutation of serine 10 to Ala in H3 led to abnormal chromosome segregation and loss during mitosis and meiosis in micronuclei 11. In addition, drugs that induced the premature phosphorylation of H3 could initiate premature chromosomal condensation in cultured cells. These researchers supposed that the conserved phosphorylation event in H3 may cause a local and transient decondensation of chromatin and a lost of interaction between histone H3 and the chromatin, thus allowing other transacting chromatin condensation factors to access and thus facilitate further chromosome condensation 12.
Another possible role of phospho-H3 is to act as a receptor to recruit chromatin condensation factors, which in turn trigger the mitotic chromatin condensation. In our previous studies, we used immunofluorescence labeling on human MCF-7 cells with antibodies specific for phospho-H3 to examine the distribution of this protein in cells, and showed that phosphorylation of histone H3 initiated in discrete foci near the nuclear envelope in early prophase cells. Dephosphorylation of H3 began in anaphase and completed immediately before chromosome consecution at telophase cells 13. Although increasing evidence indicates the relationship between phospho-H3 and mitosis, the specific patterns of histone modification have yet be generated and tested in vivo. In present study, the dynamic distribution of phospho-H3 has been examined in MCF-7 and CHO cells using indirect immunofluorescence staining with an antibody against H3 phosphorylated at Ser10. The result shows that the phosphorylation of Ser10 is a key step in chromosome condensation. Our results also demonstrate that some of the Ser10 phospho-H3 is not co-localized with chromosomal DNA during prophase and metaphase, and Ser10 phospho-H3 aggregated at midbody region as a separating factor to be engaged in cytokinesis. The result of fluorescent microscopy showed that some of Ser10 phosphorylated H3 and microtubules are compacted in the center between two sets of the separating chromosomes, forming into a sandwich-like structure at anaphase, and then a dense bar-like complex, to separate two daughter cells. The special contribution and the featured structure suggest that phospho-H3 possess the particular function in cytokinesis.
MATERIALS AND METHODS
Cell culture and histone extraction
Two mammalian cell lines were employed in this study: MCF-7 (cells from human breast adenocarcinoma) and CHO (Chinese hamster origin). All cell lines were cultured in modified RPMI 1640 medium, supplemented with 10% FCS and 1% streptomycin. Cultures were maintained in a humid chamber at 37°C with 5% CO2.
Cells were synchronized by adding Nocodazole to the media of cell culture to a final concentration of 0.6 μg/ml. After 18 h, mitotic cells were collected by mechanical shaking, and other cells by digesting with 0.25% pancreatin as interphase cells as described 14. Cells were centrifuged, the medium is discarded and the cells were washed with PBS (pH 7.4) for two times. As described previously 14, the cells were suspended in 5-10 volumes of lysis buffer (10 mM Hepes, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT and 1.5 mM PMSF). The samples were incubated on ice for 30 min and centrifuged at 1,000 g for 10 min at 4°C. The supernatant was discarded and the histones in the pellets were extracted by 0.2 M HCl solution. Then the samples were centrifuged at 12,000 g for 10 min at 4°C after incubating on ice for 30 min. Then histones were precipitated from acid solution with 5 volumes of cold acetone.
SDS-PAGE and Western blotting
The specificity of antibody used in this report was analyzed by Western blotting. The samples of acid soluble proteins were seperated on 15% SDS-PAGE. The proteins in another parallel gel were transferred onto a PVDF membrane, and then the membrane was blocked in 5% nonfat milk in TBS for 1 h and incubated with the rabbit anti-pH3 antibody (Upstate Biotechnology, NY, USA) for another 1 h. After washing, the blots were probed with horseradish peroxidase-conjugated secondary antibody and visualized by diaminobenzidin (DAB, Goldenbridge Biotechnology, Beijing, China).
Immunofluorescence
The CHO and MCF-7 cells were cultured on glass cover slips for 2 d. The cells were fixed in 4% paraformaldehyde at room temperature for 10 min, washed once with TBS, and then incubated in 0.25% TritonX-100 for 5 min at room temperature. To avoid nonspecific antibody binding, the cells were incubated for 45 min in blocking solution (10% normal goat serum in PBS) at room temperature. The specimens were subsequently washed once in TBS for 5 min, and incubated with the primary antibody (diluted in 1:200) in a humid chamber at 4°C overnight. The slides were washed 3 times for 5 min each with PBS at room temperature, and then incubated with the FITC-conjugated goat anti-rabbit IgG for 45 min at 37°C. After final three washes in PBS, the cells on the coverslips were counterstained with propidium iodide (PI) and mounted.
Co-detection of Ser10 of phospho-H3 and tubulin with three coulor fluorescent image
A succession labeling method was performed for co-detection of phosphorylated histone H3 and α-tubulin. The cells were first incubated with anti-pH3 antibodies. After washing, the goat anti-rabbit IgG-FITC antibody was added at dilution of 1:100. Then the mouse anti-α tubulin antibody (Zymed Laboritories, CA, USA) was used to perform the second staining at a dilution of 1: 50 in 1% BSA in PBS for 30 min at 37°C. The cells were washed again, and the goat anti-mouse IgG-TRITC was used to stain the microtubules in the cells at a dilution of 1:50. After final washing, the specimens were counterstained with DAPI as DNA indicator.
Confocal microscopy
Confocal scanning microscopy was performed using a TCS-NT Leica microscope (Lasertechnichk, Heidelberg, Germany). An argon-krypton ion laser was adjusted at an excitation wavelength of 345 nm, 488 nm and 568 nm. Fluorescent images were captured in sequential mode. Serial optical sections were taken. Selected paired sections were then processed to produce single composite, color-merged overlay images.
RESULTS
SDS-PAGE and Western blot analysis of phosphorylated histone H3
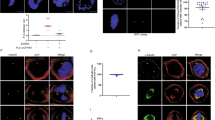
SDS-PAGE and Western blot analysis were carried out to determine the cell histone components at interphase and metaphase. As shown in Fig. 1, the acid soluble protein samples extracted from synchronized interphase and metaphase cells of MCF-7 were separated into a group of bands by electrophoresis. The core histones H2A, H2B, H3, H4 bands line up on the range of molecular weight 14.4 kD-20.1 kD (Fig. 1A). Western blot analysis show that there was no band found in column I, and only one band in column M that is Ser10 phospho-H3 detected by the antibody against Ser-10 phospho-H3 (Fig. 1B). The results indicate the antibody is highly selective for Ser-10 phospho-H3, and the H3 phosphorylation is a special event in mitosis of the cells.
Distribution of phosphorylated histone H3 in MCF-7 cells during mitosis
To determine the dynamic distribution of phospho-H3 in the cells during mitosis, chromosomal DNA was stained by PI to show the cell phase, and phospho-H3 in the cell was stained by the antibody which recognized Ser10 phospho-H3, then treated by FITC conjugated goat anti-rabbit IgG (see “Materials and Methods”). The samples were examined by confocal laser microscopy (Fig. 2). At prophase the chromosomes begin to condense, as shown on the Fig. 2A, the nuclear were filled with the fluorescent signal of phospho-H3, indicating that phosphorylation occurred at all along with chromosomes in the cell. At metaphase (Fig. 2B) all chromosomes compacted on the equatorial plate and shaped as cylinder-like entity. The green fluorescent signals of the phospho-H3 at both ends of the cylinder were much stronger than that at the center. Some of strong fluorescent staining was localization out of the end of the cylinder of the condensed chromosome; namely, the phosphorylated H3 location is not overlapping with condensed chromosome. When sister chromosomes were getting apart after centromeric fission, the cells enter anaphase. There is no fluorescent signal of phospho-H3 in the separating chromosomes (see Fig. 2C). To our surprise, the intense immunofluorescent signals appeared as punctate aligning in the midbody region, and the aggregates of phospho-H3 is composed of “sandwich-like” structure with two sets of separated chromosomes at this phase. At telophase, the separated chromosomes started to uncoil and became less condensed, immunofluorescence at midbody was as bright as before, but getting shorter and shorter during cytokinesis (Fig. 2D-F). With further de-condensation of the chromosomes, the immunofluorescent dots that presumably touched the growing tip of cleavage furrow primarily disappeared, while the green dots in the middle remained until cytokinesis completed and two independent daughter cells formed. These observations indicate that the dynamic distribution of phospho-H3 in the cells is correlated with cytokinesis progress temporally and spatially.
Dynamic distribution of Ser10 phosphorylated Histone H3 at different stages of mitosis in MCF-7 cells. (A) late-prophase; (B) metaphase; (C) anaphase; (D-E) telophase; (D-G) cytokinesis. Column PI, chromosomal DNA stained by PI; Column FITC, ser10 phosphorylated histone H3 stained by immuno-fluorescence FITC conjugated antibody; Column Merge, merged image of the PI and FITC staining.
Distribution of Ser10 phospho-H3 in CHO cells during mitosis
To confirm the ordered distribution pattern of phospho-H3 in mammalian cytoplasm (nuclear-cytoplasm merge together when nuclear envelope dispersed) during mitosis, the CHO cells were subjected to immunofluorescent examination. The results showed that phosphorylated histone H3 appeared at pericentromeric region of the nuclear at prophase (Fig. 3A). This was just the same to other research results before 10. Then CHO cells exhibited intense staining at opposite ends of the condensed cylinder of the chromosomes arranged in equatorial plate at metaphase and it shown similar pattern to MCF-7 cells (Fig. 3B). The intense fluorescent of phospho-H3 occurred in the midbody region between two sets of separated chromosomes at early anaphase (Fig. 3C), the green fluorescent signal aligned as ladder in the middle of the “sandwich-like” structure. Following the cell phase progression, the staining signal developed as punctate as MCF-7 (see Fig. 3D), and disappeared along with chromosome de-condensation (Fig. 3E-F). The results showed that the similar dynamic distribution at mitosis in two mammalian cell lines. Phosphorylated histone H3 was included in the midbody region at cytokinesis in CHO cells.
Dynamic distribution of Ser 10 phosphorylated histone H3 at different stages of mitosis in CHO cells. (A) prophase; (B) metaphase; (C) anaphase; (D) telophase; (D-F) cytokinesis. Column PI, chromosomal DNA stained by PI; column FITC, ser10 phosphorylated histone H3 stained by immuno-fluorescence FITC conjugated antibody; column merge, merged image of the PI and FITC staining. The “sandwich-like structure” is shown in C and D as the “merged” image.
Ser10 phospho-H3 and microtubule in the cytokinesis
In order to examine the dynamic distribution of phospho-H3 and microtubule in different phases of cells, three fluorescent labeling technique was performed with DAPI for DNA (blue), indirect immunofluorescence labeling for phospho-H3 (the secondary antibody conjugate to FITC, green) and α-tubulin (the secondary antibody conjugate to TRITC, red) in MCF-7 cell. The results were exhibited in Fig. 4.
The dynamic distribution of Ser10 phosphorylated histone H3 and microtubules in MCF-7 during mitosis. DNAs were stained with DAPI (blue); Ser10 phosphorylated histone H3 and microtubule were stained by indirect fluorecent labeling with FITC (green) and TRITC (red) respectively. (A) prophase; (B) metaphase; (C) anaphase; (D) telophase; (D-G) cytokinesis.
The Ser10 phospho-H3 started at early prophase of the cell (Fig. 4A). At metaphase (Fig. 4B), the condensed chromosomes arranged at equatorial plate of the mitotic spindle, but the green fluorescence signal of phospho-H3 was mostly aggregated at the ends of condensed cylinder of the chromosomes. At early anaphase, when the condensed chromosomes moved to the opposite poles of the spindle, some of phospho-H3 accumulated in the midbody region as ladder aggregates and creates a sandwich like structure with two sets of separating chromosomes. At anaphase (Fig. 4D), following the chromosome de-condensation, the ladder-like phospho-H3 changes to the punctate line up between the chromosomes. At this phase (Fig. 4D), the spindles began to disassemble, some of the remaining microtubule with the phospho-H3 aligned in parallel in midbody region. Following cleavage furrow progress, microtubule and phosph-H3 complex was compacted into a bar-like separating complex between two daughter cells (Fig. 4E and F). The phospho-H3 became a dot of the aggregate connected the two bunches of compact microtubules (Fig. 4F). The dividing cells healed the plasma membrane along with the ends of bar like separating complex and finished cytokinesis, left the microtubule-Ser10 phospho-H3 complex out of daughter cells (Fig. 4F). Obviously microtubule and Ser10-phosphorylated histone H3 play crucial roles in cytokinesis.
DISCUSSION
In present study, we used indirect immunofluorescent labeling and confocal laser microscopy to examine the dynamic distribution of phospho-H3 in mammalian cell at mitosis. The result shows that Ser10 phosphorylation of histone H3 is temporally and spatially related to mitosis of the cell . The Ser10 phosphorylation of histone H3 started at early prophase of MCF-7 and CHO cells, and reached maximum at late prophase. The phosphorylation event of histone at Ser10 is present throughout of whole genome of mitotic cell at late prophase. Our results show that the ser10 phosphorylation signal is not co-localized with condensed chromosome DNA at late prophase and metaphase. Particularly, the Ser10 phospho-H3 aggregates at the end of condensed cylinder of the chromosomes which had been arranged in equatorial plate, raising the hypothesis that the phosphorylated histone H3 might result in an increasing negative charge that make it dissociate from chromosomal DNA, therefore causing chromatin remolding and condensation.
The different histone modifications and the corresponding enzymatic systems have been reviewed extensively 7. As described in the “histone code” hypothesis 15, the modification changes the charges density on the histone tail which in turn modulates histone interactions with DNA, non-histone protein and other histones. Each site of modification serves a distinct function 16.
Histone H3 phosphorylation as a kind of modification is thought to be essential for chromatin condensation during cell mitosis and meiosis. But accumulating evidence suggested that besides chromatin compaction, H3 phosphorylation also affects the formation process 17, 18, 19. But, how to explain the regular distribution of phosphorylated histone H3 in midbody during anaphase and telophase in mammal cells?
Our results suggest that the phospho-H3 in the midbody region may come from condensed chromosomes. Phosphorylation of Ser-10 is proposed to neutralize the positive charges of the N-terminal of histone H3, and thus weakens histone tail-DNA interactions and cause whole or part of histone H3 to dissociate from DNA and enter cytoplasm, leading to chromosome remodeling and condensing 7, 20, 21. The phospho-H3 is collected in the midbody and lines up between two sets of separated chromosomes at anaphase and telophase, which is supposed to play a role as physical separating factors contributing to cytokinesis along with chromosome de-condensation. Some of the phosphorylated histone would get dephosphorylated and reconstruct normal nucleosome with DNA again, and help the cells enter interphase after mitosis.
The other possibility is that the free histone H3 in cytoplasm may be modified by phosphorylation to get involved in cytokinesis. Inner centromere protein (INCENP) and Aurora-B (B-type Aurora kinase) are the members of chromosomal passenger proteins. They are co-localizated with each other during mitosis: they spread all over the chromosomes at prophase, localize to the centromere region at metaphase, redistribute to the central region of spindle and then focus on the midbody during cytokinesis 22, 23, 24, 25. Previous evidences suggest that INCENP participates in targeting and recruiting Aurora-B. Moreover, it has been demonstrated that Aurora-B can efficiently phosphorylated H3 ser10 both in vitro and in vivo 26. In our results it seems that phospho-H3 is present in the midbody region concurrent with INCENP and Aurora-B. So we would propose that at anaphase and telophase H3 kinase such as Aurora-B could be recruited by INCENP to the central region of spindle and the midbody region, where they could phosphorylate the free histone H3 in cytoplasm.
References
Karnberg KD . Chromatin structure: a repeating unit of histomes and DNA. Science 1974; 184:868–74.
Lewin B . Nucleosome Gene VII. 7th Edition. Oxford university press, 2000; 567–615.
Bradbury EM, Inglis RJ, Matthews HR, Sarner N . Phosphorylation of very-lysine-rich histone in Physarum polycephalum. Correlation with chromosome condensation. Eur J Biochem 1973; 33:131–9.
Gurley LR, Walters SA, Tobey RA . Cell cycle-specific changes in histone phosphorylation associated with cell proliferation and chromosome condensation. J Cell Biol 1974; 60:356–64.
Ohsumi K, Katagiri C, Kishimoto T . Chromosome condensation in Xenopus mitotic extracts without histone H1. Science 1993; 262:2033–5.
Shen X, Yu L, Weir JW, Gorovsky MA . Linker histones are not essential and affect chromatin condensation in vivo. Cell 1995; 82:47–56.
Hendzel MJ, Wei Y, Mancini MA, et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincide with mitotic chromosome condensation. Chromosoma 1997; 106:348–60.
Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD . Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahmena. Proc Natl Acad Sci USA 1998; 95:7480–4.
Gurley LR, Danna JA, Barhan SS, Deaven LL, Tobey RA . Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem 1978; 84:1–5.
Wei Y, Yu LL, Bowen J, Gorovsky MA, Allis CD . Phosphosylation of histone H3 is require for proper chromosome condensation and segregation. Cell 1999; 97:99–109.
Binder A, Bohm L . Influence of irradiation and pentoxifylline on histone H3 phosphorylation in human tumour cell lines. Cell Prolif 2002; 35:37–47.
Koshland D, Strunnikov A . Mitotic chromosome condensation. Annu Rev Cell Dev Biol. 1996; 12:305–33.
Yang Q, Chen JT, Geng ZH, Yu XD, Huang XT . Studies of distribution of phosphorylated H3 in human MCF-7 cells by immunofluroescence labeling. Acta Genetica Sinica 2002; 29:471–5 (Article in Chinese)
Goto H, Tomono Y, Ajiro K, et al. Identification of a noval phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem 1999; 274:25543–9.
Strahl BD, Allis CD . The language of covalent histone modifications. Nature 2000; 403:41–5.
Van Hooser A, Goodrich DW, Allis CD, Brinkley BR, Mancini MA . Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation. J Cell Sci 1998; 111:3497–506.
de La Barre AE, Gerson V, Gout S, et al. Core histone N-termini play an essential role in mitotic chromosome condensation. EMBO J 2000; 19:379–91.
Hsu JY, Sun ZW, Li X, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 2000; 102:279–91.
Speliotes EK, Uren A, Vaux D, Horvitz HR . The survivin-like C. elegans BIR-1 protein acts with the Aurora-like kinase AIR-2 to affect chromosomes and the spindle midzone. Mol Cell 2000; 6:211–23.
Roth S, Allis CD . Chromatin condensation: does histone H1 dephosphorylation play a role? Trends Biol Sci 1992; 17:93–8.
Waring P, Khan T, Sjaarda A . Apopotosis induced by gliotoxin is preceded by phosphorylation of histone H3 and enhanced sensitivity of chromatin to nuclease digestion. J Biol Chem 1997; 272:17929–36.
Adams RR, Wheatleya SP, Gouldsworthy AM, et al. INCENP binds the aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol 2000; 10:1075–8.
Kaitna SM, Mendoza M, Jantsch-Plunger V, Glotzer M . Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol 2000; 10:1172–81.
Adam RR, Eckley DM, Vagnarelli P, et al. Human INCENP colocalizes with the Aurora-B/AIRK2 kinase on chromosomes and is overexpressed in tumour cells. Chromosoma 2001; 110:65–74.
Giet R . and Glover DM . Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J Cell Biol 2001; 152:669–82.
Crosio C, Fimia GM, Loury R, et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian aurora kinases. Mol Cell Biol 2002; 22:874–85.
Acknowledgements
We thank Zhou WM (Institute for viral disease control and prevention, Beijng) for providing the confocal microscopy and technical assistance and Dr. Cao YJ for reading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LI, D., YANG, Q., CHEN, J. et al. Dynamic distribution of Ser-10 phosphorylated histone H3 in cytoplasm of MCF-7 and CHO cells during mitosis. Cell Res 15, 120–126 (2005). https://doi.org/10.1038/sj.cr.7290276
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290276
Keywords
This article is cited by
-
Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce
Nature Medicine (2021)
-
Differential cellular proliferation underlies heterochronic generation of cranial diversity in phyllostomid bats
EvoDevo (2020)
-
Disruption in murine Eml1 perturbs retinal lamination during early development
Scientific Reports (2020)
-
Regionalized cell proliferation in the symbiont-bearing gill of the hydrothermal vent mussel Bathymodiolus azoricus
Symbiosis (2020)
-
Hsp90 and hepatobiliary transformation during sea lamprey metamorphosis
BMC Developmental Biology (2015)