ABSTRACT
Mitsugumin 29 (MG29) is a transmembrane protein that is normally found in the triad junction of skeletal muscle. Our previous studies have shown that targeted deletion of mg29 from the skeletal muscle resulted in abnormality of the triad junction structure, and also increased susceptibility to muscle fatigue. To elucidate the basis of these effects, we investigated the properties of Ca2+-uptake and -release in toxin-skinned Extensor Digitorium Longus (EDL) muscle fibers from control and mg29 knockout mice. Compared with the control muscle, submaximal Ca2+-uptake into the sarcoplasmic reticulum (SR) was slower and the storage of Ca2+ inside the SR was less in the mutant muscle, due to increased leakage process of Ca2+ movement across the SR. The leakage pathway is associated with the increased sensitivity of Ca2+/caffeine -induced Ca2+ release to myoplasmic Ca2+. Therefore, the increased fatigability of mutant EDL muscles can result from a combination of a slowing of Ca2+ uptake, modification of Ca2+-induced Ca2+ release (CICR), and a reduction in total SR Ca2+ content.
Similar content being viewed by others
INTRODUCTION
In skeletal muscle, a functional unit of excitation-contraction coupling (ECC) involves the triad junction formed by the transverse tubule and junctional SR membranes, where the dihydropyridine receptor (DHPR) and the ryanodine receptor (RyR) proteins interact with each other 1. The DHPR works as the voltage sensor across the sarcolemmal membrane 2 and the RyR functions as the Ca2+ release channel in the SR 3, 4. A number of accessory proteins (e.g. calmodulin, FKBP12, triadin, sorcin, junctin, calsequestrin) have been identified and proposed to be involved with the regulation and modulation of the RyR Ca2+ release channel 5.
Mitsugumin 29 (MG29), a transmembrane protein expressed in the triad junction of skeletal muscles 6, has also been proposed to participate in the ECC process of skeletal muscle 7, 8. The primary amino acid sequence of MG29 exhibits a high degree of homology to synap-tophysins, a group of membrane proteins with presumed roles in neurotransmitter release 9. It has been shown 7 that while mg29 knockout mice survive, reproduce, and show no abnormalities of health under normal housing conditions, extensor digitorum longus (EDL) muscles isolated from the knockout mice produced a slightly lower twitch force but equal peak tetanic force when compared with muscles from wild-type mice. A hallmark feature of skeletal muscles from these mutant mice is the presence of morphological abnormalities of membrane structures around the triad junction, characterized by swollen T-tubule and fragmented SR network 7. This observation suggested that MG29 is not only important for the proper organization of both the intracellular and cell surface membranes, but also for signal transduction in ECC.
We have previously tested whether the defective membrane structures in the mg29 knockout mice have an impact on the susceptibility of skeletal muscles to fati-guing stimulation 8. We compared the contractility and fatigability of intact skeletal muscles isolated from the MG29 knockout mice with those from wild type (control) mice, and identified three phenotypes: a) an increased rate of fatigue development in EDL muscle, b) an increased extent of fatigue in EDL and soleus (SOL) muscles, and, c) a lesser recovery of force in EDL and SOL muscles after fatigue.
To elucidate the cellular basis for the three phenotypes in EDL muscles from mg29 knockout mice, we investigated the effects of caffeine on the isometric contractile properties of intact muscles during the recovery from fatiguing stimulation. We also measured Ca2+ uptake, Ca2+/caffeine -induced Ca2+ release and total Ca2+ release properties of α-toxin-skinned EDL muscle fibers from control and mg29 knockout mice. Intact EDL muscles from mg29 knockout mice recovered significantly less than control muscles even in the presence of high concen-trations of caffeine. In skinned muscle fibers, we found that submaximal Ca2+ uptake is slowed and Ca2+/caffeine -induced Ca2+ release is modified in such a way to suggest that it was dramatically enhanced in mutant muscles and total Ca2+ stored within the SR is significantly less, suggesting a leakier SR in mutant muscles.
MATERIALS AND METHODS
Generation of mutant mice
The procedure for generation of mg29 knockout mice used in this study has been published elsewhere 7. These mice were generated from the C57BL/6J background. For comparative studies, the SV129/J x C57BL6 strain was used as the wild-type control. Care was taken to ensure that all procedures relating to the living animals were in accordance with the “Guiding Principles in the Care and Use of Animals” approved by the American Physiological Society.
Intact muscle preparation
The detailed experimental protocol for isolation of intact muscle from mice has been described by Brotto et al 10. Briefly, pairs of intact EDL muscles, 1 control, and 1 mutant, were mounted vertically on two Radnoti (Monrovea, CA) glass apparati with platinum stimulating electrodes. The isometric contractile force of the intact muscles was monitored on a strip chart recorder, digitized and stored for later analysis. After muscles were mounted, the resting tension, current and frequency of stimulation (∼ 300 mA and 100 Hz.) were adjusted to produce maximal tetanic force (i.e., Tmax). The twitch force and Tmax were normalized to force/cross sectional area by using the following relationship: F/cm2= [force (g)*muscle length*1.06]/muscle weight. To follow the time course and recovery from fatiguing stimulation, all force data were normalized to the Tmax event measured just prior to the fatiguing protocol. Force versus frequency data was normalized to the maximum force generated by each muscle. Upon completion of an experimental protocol, the muscles were removed from the bathing medium and rapidly transferred to the skinning solution described below.
α-Toxin-skinned Muscle Fibers
These experiments followed the protocols described in Brotto and Nosek 11 as recently modified in Laukinobis and Stephenson 12. Small bundles of muscle fibers (5-10 fibers), were exposed for 30 min to 5 μM α-toxin. In this study, we utilized α-toxin-skinned muscle fibers for the SR Ca2+-uptake and -release experiments, instead of saponin-skinned muscle fibers, because Laukinobis and Stephenson 12 have recently demonstrated that saponin and β-escin may exert deleterious effects on the SR membrane. In agreement, we have found that caffeine responses are more robust, reproducible and consistent in α-toxin-skinned muscle fibers when compared with saponin-skinned muscle fibers. Muscle fibers were mounted between an optoelectric force transducer (Scientific Instruments GMBH, Heidelberg, Germany) and a movable arm by wrapping the fibers 3 times around small stainless steel wires. Only a very small part (∼ 300-500 μm) of the fiber remained free in between the two arms and the muscle fibers were stretched by 30% (sarcomere length ∼ 2.4–2.6 μm). The fibers were bathed in solutions contained in 2.5 ml troughs milled in a spring-loaded Plexiglas plate. Single muscles were subsequently exposed to the solutions that comprised the specific protocols for SR Ca2+-Uptake, Ca2+/caffeine -induced Ca2+ release and total Ca2+ content of the SR.
SR Ca2+-uptake
For all the experiments in which we tested SR function, each fiber was initially incubated in the wash solution, loaded with Ca2+ for 10 s and exposed to caffeine. The majority (∼85-90%) of the fibers reproducibly responded to caffeine, while less than 50% responded when skinned with saponin in this pre-test phase in agreement with the findings of Leukanobis and Stephenson 12. Muscle fibers are changed from one solution to the other within 1-2 s and are incubated in each solution as follows: first, they were equilibrated in a relaxing solution (1.0 Mg2+; 5.0 MgATP; 15 phosphocreatine; 140.0 KMS; 50.0 imidazole, 200 ionic strength, 5.0 EGTA, pCa < 8.5, pH 7.1), then the SR was depleted of all Ca2+ releasable with caffeine (1.0 Mg2+; 5.0 MgATP; 15 phosphocreatine; 140.0 KMS; 50.0 imidazole, 200 ionic strength, 5.0 EGTA, pCa < 8.5, pH 7.1 and 25 mM caffeine), the SR was then loaded with Ca2+ (1.0 Mg2+; 5.0 MgATP; 15 phosphocreatine; 140.0 KMS; 50.0 imidazole, 200 ionic strength, 5.0 EGTA, pCa 6.0, pH 7.1) and the amount of Ca2+ loaded into the SR was probed by the contracture response induced by a caffeine-induced Ca2+ release solution (0.1 Mg2+; 0.1 MgATP; 5 phospho-creatine; 140.0 KMS; 50.0 imidazole, 200 ionic strength, 0.1 EGTA, pCa < 8.5, pH 7.1, 25 mM caffeine). From these experiments, a relationship (% of SR Ca2+ loading (obtained from the graded caffeine contractions) vs. Loading time (in s) was obtained and from this curve, the time to load the SR by 50% was calculated.
Ca2+/caffeine -induced Ca2+ release
The initial three steps were identical to those used for the SR Ca2+-uptake protocol and then the muscle fibers were incubated for a fixed amount of time (30 s) in the loading solution. The fibers were then incubated for 2 min in a rigor solution (1.0 Mg2+; 0.0 MgATP; 140.0 KMS; 50.0 imidazole, 200 ionic strength, 1.0 EGTA, pCa < 8.5, pH 7.1) to inhibit the SERCA pump and prepare the internal milieu for the CICR phase, which comprised of a 30 s exposure to either the control (zero Ca2+) or the CICR solution (0.1 Mg2+; 0.0 MgATP; 140.0 KMS; 50.0 imidazole, 200 ionic strength, 0.2 EGTA, pCa 5.0, pH 7.1), followed by a 30 s exposure to a solution designed to stop the release of Ca2+ from the SR (10.0 Mg2+; 0.0 MgATP; 140.0 KMS; 50.0 imidazole, 200 ionic strength, 0.2 EGTA, pCa 5.0, pH 7.1). The last three steps of the CICR protocol were also identical to the last three steps used for the SR Ca2+-uptake protocol. Note that each muscle fiber passes through this cycle three times. In the first run, they are exposed to pCa 8.5 solution. On the second to pCa 5.0 solution and on the third run to pCa 8.5 solution. Because pCa 8.5 solution has nominal Ca2+ equal to zero, no Ca2+ is expected to be released except any Ca2+ that may passively leak from the SR due to the low Mg2+ concentration in this solution. The caffeine responses after the two bracketed exposures of the muscle fibers to this control solution (pCa 8.5) are normalized to 100%, which means that 100% of Ca2+ was left within the SR. During the second run, the muscle fibers are exposed to pCa 5.0 and their response to caffeine after this exposure is decreased, indicating that significantly less Ca2+ was left within the SR as Ca2+ was released during the exposure to the pCa 5.0 solution. This difference between the average of the two-bracketed responses after 8.5 and the response after pCa 5.0 is taken as Ca2+/caffeine -induced Ca2+ release index.
Ionomycin-induced Ca2+ release (IICR)
In a separate series of experiments, we exposed the muscle fibers to 5 μM ionomycin after they had been exposed to caffeine. The goal was to test whether or not control and mutant muscles displayed any differences in total SR Ca2+ content. The pre-test phase and the initial three steps were identical to those used for the Ca2+/caffeine -induced Ca2+ release experiments. After loading the fibers for 30 s, they were exposed to the caffeine-induced Ca2+ release solution. The fibers were then transferred to a relaxing solution and then exposed a solution with the composition identical to the caffeine-induced Ca2+ release solution, except that caffeine was replaced with 5 μM ionomycin. Ionomycin responses were normalized to the caffeine response obtained in the same fiber before exposure to ionomycin. Exposure to ionomycin rendered the muscle fibers unresponsive to further stimulation by either caffeine or ionomycin. The combination of the responses to caffeine and ionomycin was interpreted as the total Ca2+ stored within the SR.
Statistics
SigmaStat (Jandel Corp.) was used to statistically evaluate all data. Data are expressed as mean ± SEM. One-way ANOVA or Kruskal-Wallis ANOVA on ranks were performed on parametric and nonparametric data, respectively, to compare differences among groups for an individual variable with a P = 0.05 accepted as the criterion for statistical significance.
RESULTS
Effects of fatiguing stimulation on intact and triton-skinned muscles
We have previously demonstrated that fast-twitch muscles of mutant mice display a phenotype characterized by increased fatigability and lesser recovery from fatigue 8. Here, we compared the effects of fatiguing stimulation on the maximal tetanic force (Tmax) of intact muscles in the presence of caffeine. Fig. 1 confirms our previous findings that intact EDL muscles from mutant animals fatigue to a greater extent (P < 0.05) than muscles from control animals. Intact muscles from mutant animals also recover to a lesser extent (P < 0.03) after a 20 min recovery period. Recovery of Tmax is complete in the control muscles only after exposure to caffeine indicating that the lack of full recovery in the control muscles is due to a deficit in the ECC process, probably due to a depression of CICR 13. In contrast, Tmax of muscles from mutant animals did not (P < 0.02) fully recover when exposed to caffeine. Even after 30 min recovery and in the presence of 34 mM caffeine, Tmax is approximately 30-40% below a full recovery level in mutant muscles, suggesting defective ECC machinery.
Altered Ca2+ uptake into the SR in mg29 knockout mice
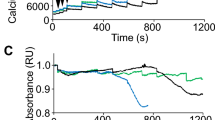
The changes in membrane structure may affect the Ca2+ recycling properties of the mutant muscle. To examine the Ca2+ transport property across the SR membrane, we used the α-toxin-skinned EDL muscle preparation. Traditionally, saponin is used to permeabilize the sarcolemma in these types of experiments. However, Launikonis and Stephenson 12 have recently demonstrated that saponin may exert detrimental effects on the SR membrane. Since α-toxin has been shown to safely permeabilize smooth muscle cells and have no apparent effects on the SR membrane 14, and we have observed that caffeine responses are more robust and reproducible in α-toxin skinned fibers, we elected to use α-toxin in this study. Fig. 2 shows that time-dependent Ca2+ uptake into the SR membrane is significantly different between the EDL muscle from control and mutant mice. At 15 s, SR loading was 76 ± 5 of the maximum loading in the control group, but significantly less in the mutant (58 ± 3%, P < 0.03). In addition, the time to achieve 50% loading of the SR was significantly longer in the mutant muscles (9 ± 1 s in control muscles vs. 13 ± 2 in mutant muscles, P < 0.03). Thus, the physiologically relevant, submaximal Ca2+ loading of the SR is significantly slower in the mutant. Notice that in both control and mutant EDL muscles loading was essentially identical at 30 s, but control muscles displayed a 15% decrease in SR loading at 45 and 60 s (P < 0.03) which was not observed in mutant EDL muscles. We believe that as the SR is near-maximally loaded with Ca2+, because CICR is functional in the control muscles, the longer exposure time to the loading solution may trigger Ca2+ release due to CICR from the SR.
SR Ca2+-uptake vs. time relationships in WT (solid squares) and mg29 knockout (open circles) EDL skinned muscle fibers. Values are expressed as averages ± SEM of the force developed in response to caffeine contractures after varied loading times (see Methods for details). All values are normalized to maximum uptake for each fiber, taken as the maximal caffeine contracture produced by each fiber. 50% loading time of the SR was achieved at significantly longer times (*P < 0.03) in mg29 knockout EDL muscles.
Ca2+/caffeine -induced Ca2+ release is modified in mg29 knockout mice
The steady-state changes in Ca2+/caffeine -induced Ca2+ release in the mutant EDL muscle were examined in the next series of studies (Fig. 3). Others and we have previously demonstrated that under experimental conditions similar to those used here, CICR is present and functional in rabbit 15, mouse 16 and rat 11 skinned muscle fibers. Endo and Takeshima 17 have also demonstrated that CICR is present and functional in skinned skeletal myocytes from mice. These authors concluded that CICR was mediated via RyR-1 and RyR-3 in mammalian skeletal muscles, because in permeabilized myocytes lacking both the RyRs, CICR mechanism was completely lost, and caffeine failed to induce Ca2+ release 15. As no significant differences for SR loading was detected at ∼ 30 s loading time for both wild type and mutant EDL muscles, this period of loading was used in these series of experiments. It is important to ensure that both groups of muscles were equally loaded, since SR loading will influence Ca2+ release activity. After this 30 s loading period, the SR was incubated for 30 s to two bracketed exposures of a zero Ca2+ solution (pCa 8.5) or a (pCa 5.0, i.e., 10 μM free Ca2+) solution. The amount of Ca2+ remaining in the SR after exposure to these solutions was assessed by measuring the contractile force produced when the skinned fibers were exposed to a combination of a high concentration of caffeine (i.e., 25 mM) and a low concentration of magnesium (i.e., 0.1 mM). These experimental conditions are expected to promote the release of all the SR Ca2+ that is releasable by caffeine 18. Furthermore, a solution containing 10 μM free Ca2+ releases Ca2+ from the SR via the CICR process in mammalian skinned muscle fibers 11, 15, 17, 19. Here, we found that Ca2+/caffeine -induced Ca2+ release in muscle fibers from mutant mice were significantly (P < 0.001) different than that displayed by muscle fibers from controls. The results of these experiments are summarized in Fig. 3.
Ca2+/caffeine -induced Ca2+ release properties of WT (white column) and mg29 knockout (white/black column) EDL skinned muscle fibers. Values are expressed as means ± SEM of the amount of Ca2+ released from the SR by exposure of the muscle fibers to a pCa 5.0 solution (i.e., 10 μM free Ca2+) as compared to a pCa 8.5 solution (i.e., zero nominal Ca2+). * means P < 0.001.
To further characterize the effects of the mutation on Ca2+ handling by the SR, we compared the ability of ionomycin to release Ca2+ from the SR after the fibers had been treated with caffeine. Sorenson et al 20 using mammalian skinned muscle fibers and SR vesicles demons-trated that caffeine increases the Ca2+ permeability in a limited population of SR membranes and that these membranes coexisted with a population of caffeine-insensitive membrane within the same fiber. In contrast, ionomycin is a Ca2+ ionophore, which allows all Ca2+ (i.e., even the Ca2+ that is not releasable by caffeine) to be released from the SR 21, 22. Muscle fibers were loaded for 30 s and then exposed to caffeine. After exposure to caffeine, muscles were allowed to relax and subsequently exposed to 5 μM ionomycin. Under these conditions the fibers from control muscles contracted to approximately 85 ± 35% of the magnitude of the contraction obtained in response to caffeine, while mutant muscles contracted to only 18 ± 15% (P < 0.001) (Fig. 4). These results suggest that total Ca2+ within the SR of muscles from mutant animals is dramatically reduced; i.e., there is defective Ca2+ handling in mutant EDL muscles, characterized by either a leaky Ca2+ pathway or an avidly Ca2+-releasable pool in the SR. Another possibility is that during the relaxation phase after exposure to caffeine, Ca2+ was re-uptaked into the SR. Since submaximal SR Ca2+ uptake is slower in mutant muscles (as shown in Fig. 2), it is possible that less Ca2+ was available to be released by ionomycin treatment in the mutant skinned fibers as compared with control fibers. In any event, we believe that from the SR Ca2+ uptake, Ca2+/caffeine -induced Ca2+ release and ionomycin experiments, it can be concluded that the SR was leakier (or less loaded with Ca2+) and also that the ECC machinery is defective in mutant muscles.
Ionomycin-induced Ca2+ release from the SR of WT and mg29 knockout EDL skinned-muscle fibers. Values are expressed as means ± SEM of the magnitude of force produced by exposure of the muscle fibers to 5 μM ionomycin. Responses were normalized to the caffeine response produced by each fiber prior to exposure to ionomycin. * means P < 0.001.
DISCUSSION
Pioneering studies by Takeshima and coworkers suggested that MG29 participates both structurally and functionally in the ECC process of skeletal muscle 6, 7. Recently, our laboratories further expanded this initial proposal by demonstrating that the absence of MG29 in fast- and slow-twitch skeletal muscles determines a phenotype characterized by an increased fatigability in these muscles 8. In an attempt to further advance our current understanding of how MG29 is involved in the ECC process under resting and fatiguing conditions, we investigated the cellular mechanisms that might lead to the phenotypes we have identified in mice lacking the mg29 gene. Therefore, we studied the effects of mg29 deletion on the contractile properties of intact muscles in the presence of caffeine and the characteristic properties of both Ca2+ uptake and CICR of α-toxin-skinned muscle fibers. We found that recovery from fatiguing was significantly lower in mutant EDL muscles even in the presence of high concentrations of caffeine.
Because Ca2+ metabolism also significantly affects force development in intact muscles, we investigated the effect of MG29 on the SR Ca2+-uptake and Ca2+/caffeine -induced Ca2+ release processes and found that EDL muscles lacking the MG29 protein display a disrupted ECC process characterized by a significantly slower Ca2+ uptake and an essential abolishment of CICR. A striking finding in our studies was the altered behavior of CICR in mutant EDL muscles. The seemingly loss of the steady-state response to CICR could result from two possible mechanisms: a) complete loss of Ca2+ sensitivity of the process, or, b) dramatically increased sensitivity to Ca2+ making an effect of the elevated Ca2+ levels used in the CICR protocol essentially ineffectual. Evidence for this latter interpretation is supported by our finding that the total Ca2+ pool of the SR (assessed by Ca2+ release in response to ionomycin after treatment with caffeine) is dramatically reduced in skinned fibers from the mg29 knockout mice. In addition, our protocols do not allow for a continuous recording of the CICR process, but rather measures the net final response after the exposure time to either a nominal zero Ca2+ solution or a solution containing 10 mM free Ca2+, and it may also reflect a combination of the responses to both Ca2+ and caffeine. Because caffeine is thought to act via a CICR-like mechanism, we conclude that the leakage pathway in mutant muscles is associated with the increased sensitivity of CICR to myoplasmic Ca2+.
Therefore, the increased fatigability of mutant EDL muscles can result from a combination of a decrease in the physiologically relevant, submaximal Ca2+ loading of the SR, modification of Ca2+/caffeine -induced Ca2+ release, and a reduction in total SR Ca2+ content. Taken together, we have demonstrated that MG29 is a major player in the cascade of events that regulate and modulate normal ECC process. Disruption of the normal triad seems to lead to a subsequent disruption of the ECC process that is ultimately responsible for the increased fatigability in skeletal muscles from MG29 null mice.
References
Franzini-Armstrong C, Jorgensen AO . Structure and development of E-C coupling units in skeletal muscle. Annu Rev Physiol 1994; 56:509–34.
Rios E, Pizarro G . Voltage sensors and calcium channels of excitation-contraction coupling. NIPS 1988; 3:223–7.
Fleischer S, Inui M . Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem 1989; 18:333–64.
Suda N, Franzius D, Fleig A, et al. Ca2+-induced Ca2+ release in Chinese hamster ovary (CHO) cells co-expressing dihydropyridine and ryanodine receptors. J Gen Physiol 1997; 109:619–31.
MacKrill JJ . Protein-protein interactions in intracellular Ca2+-release channel function. Biochem J 1999; 337(Pt 3):345–61.
Takeshima H, Shimuta M, Komazaki S, et al. Mitsugumin29, a novel synaptophysin family member from the triad junction in skeletal muscle. Biochem J 1998; 331(Pt 1):317–22.
Nishi M, Komazaki S, Kurebayashi N, et al. Abnormal features in skeletal muscle from mice lacking mitsugumin29. J Cell Biol 1999; 147: 1473–80.
Nagaraj RY, Nosek CM, Brotto MA, et al. Increased susceptibility to fatigue of slow- and fast-twitch muscles from mice lacking the MG29 gene. Physiol Genomics 2000; 4:43–9.
Llona I . Synaptic like microvesicles: do they participate in regulated exocytosis? Neurochem Int 1995; 27:219–26.
Brotto MA, Andreatta-van Leyen S, Nosek CM, Brotto LS, Nosek TM . Hypoxia and Fatigue-induced Modification of Function and Proteins in Intact and Skinned Murine Diaphragm Muscle. Pflugers 2000; 440:727–34.
Brotto MA, Nosek TM . Hydrogen peroxide disrupts Ca2+ release from the sarcoplasmic reticulum of rat skeletal muscle fibers. J Appl Physiol 1996; 81:731–7.
Launikonis BS, Stephenson DG . Effects of beta-escin and saponin on the transverse-tubular system and sarcoplasmic reticulum membranes of rat and toad skeletal muscle. Pflugers 1999; 437:955–65.
Brotto LS, Brotto MA, Nosek CM, Nosek TM . The effects of fatiguing stimulation on calcium-induced calcium release (CICR) of skinned muscle fibers. Biophys J 2001;80:379.
Kitazawa T, Kobayashi S, Horiuti K, Somlyo AV, Somlyo AP . Receptor-coupled, permeabilized smooth muscle: Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem 1989; 264:5339–42.
Ikemoto T, Iino M, Endo M . Enhancing effect of calmodulin on Ca2+- induced Ca2+ release in the sarcoplasmic reticulum of rabbit skeletal muscle fibres. J Physiol 1995; 487:573–82.
Thompson VF, Lawson K, Goll DE . Effect of mu-calpain on m-calpain. Biochem Biophys Res Commun 2000; 267:495–9.
Ikemoto T, Komazaki S, Takeshima H, et al. Functional and morphological features of skeletal muscle from mutant mice lacking both type 1 and type 3 ryanodine receptors. J Physiol 1997; 501:305–12.
Lamb GD, Cellini MA, Stephenson DG . Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol 2001; 531:715–28.
Ikemoto T, Takeshima H, Iino M, Endo M . Effect of calmodulin on Ca2+-induced Ca2+ release of skeletal muscle from mutant mice expressing either ryanodine receptor type 1 or type 3. Pflugers Arch 1998; 437:43–8.
Sorenson MM, Coelho HS, Reuben JP . Caffeine inhibition of calcium accumulation by the sarcoplasmic reticulum in mammalian skinned fibers. J Membr Biol 1986; 90:219–30.
Bhogal MS, Colyer J . Depletion of sarcoplasmic reticulum calcium prompts phosphorylation of phospholamban to stimulate store refilling. Ann N Y Acad Sci 1998; 853:260–3.
Toth A, Ivanics T, Ruttner Z, et al. Quantitative assessment of [Ca2+]i levels in rat skeletal muscle in vivo. Am J Physiol 1998; 275:H1652–62.
Acknowledgements
This work was supported by the NIH-NIA Minority Faculty Training Grant and Supplemental Grant Award (AG-15556) (to M. Brotto and J. Ma, respectively), The American Heart Association (Ohio Valley Affiliate) postdoctoral fellowship (to R. Y. Nagaraj), by the National Institutes of Health Grant HL-60304 (to T. M. Nosek and M. Brotto) and grants AG-15556 and CA-95739 (to J. Ma).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
BROTTO, M., NAGARAJ, R., BROTTO, L. et al. Defective maintenance of intracellular Ca2+ homeostasis is linked to increased muscle fatigability in the MG29 null mice. Cell Res 14, 373–378 (2004). https://doi.org/10.1038/sj.cr.7290237
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290237
Keywords
This article is cited by
-
A focus on extracellular Ca2+ entry into skeletal muscle
Experimental & Molecular Medicine (2017)
-
Mitsugumin 29 regulates t-tubule architecture in the failing heart
Scientific Reports (2017)
-
Predicting muscle fatigue: a response surface approximation based on proper generalized decomposition technique
Biomechanics and Modeling in Mechanobiology (2017)
-
T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases
Skeletal Muscle (2011)