ABSTRACT
In order to study the mechanism of the effect of heparin on apoptosis in carcinoma cells, the nasopharyngeal carcinoma cell line CNE2 was used to identify the effect of heparin on apoptosis associated with the expression of c-myc, bax, bcl-2 proteins by use of Hoechst 33258 staining, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL), agarose gel electrophoresis, and flow cytometry, as well as Western blot analysis. The results showed that heparin induced apoptosis of CNE2 cells including the morphologic changes such as reduction in the volume, and the nuclear chromatin condensation, as well as the “ladder pattern” revealed by agarose gel electrophoresis of DNA in a concentration-dependent manner. The number of TUNEL-positive cells was dramatically increased to 33.6±1.2% from 2.8±0.3% by treatment with heparin in different concentrations (10 ∼ 40 kU/L). The apoptotic index was increased to 32.5% from 3.5% by detecting SubG1 peaks on flow cytometry. Western blot analysis showed that levels of bcl-2, bax and c-myc were significantly overexpressed by treatment with the increase of heparin concentrations. These results suggest that heparin induces apoptosis of CNE2 cells, which may be regulated by differential expression of apoptosis-related genes.
Similar content being viewed by others
INTRODUCTION
Heparin is a polysulfated glycosaminoglycan with a high negative charge. Heparin is synthesized in various tissues, especially in the liver, lung, and gut. In addition to its anti-coagulant activity, heparin is known to have anti-hypertensive1, anti-inflammatory 2, and anti-proliferative effects. Besides, heparin inhibits leukocyte rolling and its adhesion to endothelium, its aggregation, degranulation, and the generation of superoxide anion by activating neutrophils3, 4, 5. Heparin and heparin sulfate proteoglycan inhibit mitogenesis and migration of cultured mesangial cells6,7. It also exerts direct actions on the resident glomerular cells.
There are many actions for heparin to exert its effects on the malignant processes. Microthrombin with its fibrin formation, that is resulted from tumors and can in turn arrest tumor cells in capillaries9, impede activity of natural killer cell8. Heparin prevents the formation of thrombin and neutralizes its activity. Heparin minimizes angiogenesis, that plays an important role in metastasis, via the inhibition of vascular endothelial growth factor and platelet activating factor. It decreases tumor cell adhesion to vascular endothelium as it inhibits actions of selectin and chemokine, and also decreases the replication and activity of some oncogenic viruses10. Heparin competitively inhibits tumor cell attachment to heparin sulfate proteoglycans, blocks the oncogenic action of ornithine decarboxylase, and enhances the antineoplastic effect of transforming growth factorβ11. Heparin inhibits activator-protein-1, that is the nuclear target of many oncogenic signal transduction pathways, and potently inhibits carcinogenic casein kinase II 12.
Although a number of biological properties have been postulated to explain the effects of heparin on the malignant process, it is still unknown whether and how heparin modulates survival of carcinoma cell. In this investigation, we address the potential effects of heparin for inducing the apoptosis of human nasopharyngeal carcinoma CNE2 cells.
MATERIALS AND METHODS
Drugs and reagents
Heparin, RPMI-1640 medium, RNase A, Agarose gel, Hoechst33258, Propidium iodide (PI), Proteinase K, were purchased from Sigma Chemical Co (St. Louis, MO, USA), Cell Death Detection Kit was obtained from Boehringer Mannheim. Anti-bax, anti- bcl-2, anti-c-myc, and anti-mouse beta-actin antibody, were purchased from Santa Cruz. CNE2 cell line was provided by Cancer Institute, Sun-Yet Sen University of Medical Sciences Guangzhou, China.
Cell culture
CNE2 cell line was cultured in RPMI-1640 medium, containing 10% new bovine serum, penicillin G (100kU/L), and kanamycin (0.1g/L) at 37°C in a 5% CO2 air atmosphere.
Hoechst 33258 Staining
Cells were fixed with 4% formaldehyde in phosphate buffered saline (PBS) for 10 min, stained by Hoechst33258 (10mg/L) for 1 h, and then subjected to fluorescence microscopy. After treatment with heparin, the morphologic changes including reduction in the volume and nuclear chromatin condensation were observed.
TUNEL
TUNEL assay was performed using the apoptosis detection system. Cells were fixed with 4% paraformaldehyde in PBS overnight at 4°C. The samples were washed with PBS and then permeabilized by 0.2% Triton X-100 in PBS for 15 min on ice. After washing, cells were equilibrated at room temperature for 15 to 30 min in equilibration buffer (200 μM potassium cacodylate, 0.2 mM dithiothreitol, 0.25 g/L bovine serum albumin, and 2.5 mM cobalt chloride in 25 mM Tris-HCl, pH 6.6) and then incubated in the presence of 5 μM fluorescein-12-dUTP, 10 μM dATP, 100 μM ethylenediaminetetraacetic acid (EDTA) and terminal deoxynucleotidyl transferase at 37°C for 1.5 h in the dark. The tailing reaction was terminated by 2×standard saline citrate (SSC). The samples were then analyzed with fluorescence microscopy. At least 1000 cells were counted, and the percentages of TUNEL-positive cells were determined.
Ladder detection assay
After induction of apoptosis, cells (5×106/sample,both attached and detached cells) were lysed with 150 μl hypotonic lysis buffer (10mM EDTA, 0.5% Triton X-100 in mM Tris-HCL, Ph7.4) for 15 min on ice and were precipitated with 2.5% polyethylene glycol and 1 M NaCl for 15 min at 4°C. After centrifugation at 16,000 g for 10 min at room temperature, the supernatant was incubated in the presence of proteinase K(0.3 g/L) at 37 °C for one hour and precipitated with isopropanol at −20°C. After centrifugation, each pellet was dissolved in 10 μl of Tris-EDTA (pH 7.6) and electrophoresed on a 1.5% agarose gel containing ethidium bromide. Ladder formation of oligonucleosomal DNA was detected under ultraviolet light.
Flow cytometry
For DNA content analysis, heparin was added to CNE2 cells in mid-logarithmic phase(1×109 cells/L). After 48 h, 1×106 cells were collected, pelleted, washed with phosphate-buffered saline (PBS), and resuspended in PBS containing 20 mg/L PI and 1 g/L ribonuclease A. 106 fixed cells were examined per experimental condition by flow cytometry, and percentage of degraded DNA was determined by the number of cells displaying subdiploid (sub-G1) DNA divided by the total number of cells examined.
Western blot analysis
The cells were lysed in lysis buffer (25 mM Hepes, 1.5% Triton X-100,1% sodium deoxycholate, 0.1 %SDS, 0.5M NaCl, 5mM EDTA, 50 mM NaF, 0.1 mM sodium vanadate, 1mM phenylmethylsulfonyl fluoride (PMSF), and 0.1 g/L leupeptin) (pH7.8) at 4°C with sonication. The lysates were centrifuged at 15,000g for 15 min and the concentration of the protein in each lysate was determined with Coomassie brilliant blue G-250. Loading buffer (42 mM Tris-HCl, 10% glycerol, 2.3% SDS, 5% 2-mercaptoethanol and 0.002% bromophenol blue) was then added to each lysate, which was subsequently boiled for 3 min and then electrophoresed on a SDS-polyacrylamidel gel. Proteins were transferred to nitrocellulose and incubated respectively with anti-bcl-2, -bax, and -c-myc antibody, and then with peroxidase-conjugated secondary antibodies. Detection was performed with enhanced chemiluminescence reagent.
Statistical analysis
Data are expressed as means±SD. Statistical analysis was performed using Student's test to compare data in different groups. A P value of < 0.05 was used to indicate the statistically significant differences.
RESULTS AND DISCUSSION
Effect of heparin on apoptosis of NPC cell line CNE2
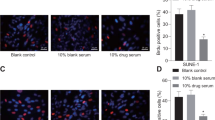
To examine the effect of heparin on the apoptosis, CNE2 cells were pretreated with heparin (10 kU/L ∼ 40 kU/L). The initiating effect of heparin on the CNE2 cell apoptosis was confirmed by observing Hoechst 33258-stained morphologic changes including reduction in the volume and nuclear chromatin condensation (not shown). Similarly, as shown in Fig 1, the number of TUNEL-positive cells was dramatically increased by the treatment with different concentrations of heparin, and the percentage of apoptosis was increased from 2.8±0.3% to 33.6±1.2%. The pro-apoptosis effect of heparin was further confirmed by the ladder detection assay and flow cytometry. Agarose gel electrophoresis showed DNA ladder formation in exposed CNE2 cells. The DNA ladder was clearly embodied by the treatment with heparin (Fig 2). Low-concentration heparin (10 kU/L) was found to be sufficient to induce DNA fragmentation. It can be observed obviously that the profiles of DNA histograms were strikingly different from untreated CNE2 cells; the subG1 peak and apoptotic index were increased from 3.5% to 32.5% (Fig 3).
DNA ladder pattern formation in CNE2 cells after treatment with heparin. Cells were treated different concentrations of heparin for 48 h and the formation of oligonucleosomal fragments was determined by 1.5% agarose gel electrophoresis. M) DNA markers; 1) control; 2) 40 kU/L heparin; 3) 20 kU/L heparin; 4) 10 kU/L heparin
Heparin has been considered as an anti-mitotic agent for several cell types, especially for vascular smooth muscle cells and glomerular mesangial cells. However, little information is available concerning the effect of heparin on apoptotic processes. It is suggested by some reports that heparin may facilitate apoptosis in human peripheral blood neutrophils and in human lymphoblasts, mononuclear cells13. On the other hand, it is also suggested that heparin inhibits apoptosis in cultured mesangial cells and explanted glomeruli14. Nevertheless, the effect of heparin on apoptotic processes has not been clearly defined in carcinoma cells, although well studied in normal cells. In this investigation, we demonstrated a novel potential effect of heparin as an inducer of apoptosis in nasopharyngeal carcinoma (NPC) cell line, CNE2 cells, which showed that heparin induces the apoptosis of CNE2 cells in a dose-dependent manner.
Enhanced expression of c-myc and the ratios of bax/bcl-2 in CNE2 cells exposed to heparin
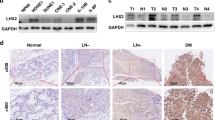
Less is known about the molecular mechanisms underlying the pro-apoptotic action of heparin. Previous researches have shown that heparin inhibits a PKC-dependent pathway for cell proliferation by suppressing the expression of c-fos and c-myc protooncogene, as well as IL-11 and GM-CSF mRNAs15 in murine fibroblasts and vascular smooth muscle cells. Heparin, that was proved to be a potent inhibitor of PKC, interacted with the catalytic domain of PKC. However, the present study, as shown in Fig 4, revealed that heparin increased the expression of bcl-2, bax, and c-myc in cultured human nasopharyngeal carcinoma (NPC) cell line CNE2.
c-myc, bcl-2 and bax protein levels in CNE2 cells treated with heparin. Cell lysates were collected and processed at 48 h. The whole cellular protein was separated in SDS-PAGE, Western blot was performed using antibodies against c-myc, bcl-2 and bax. Beta actin was used as a lane-loading control. 1) control; 2) 10 kU/L heparin; 3) 20 kU/L heparin; 4) 40 kU/L heparin.
It depends upon the interaction of other signals and related genes as whether or not c-myc in a particular cell type will promote cell proliferation and differentiation or induces apoptosis. The c-myc-mediated apoptosis could be suppressed by cytokines such as platelet-derived growth factor (PDGF), insulin-like growth factor-I (IGF-I), and bcl-2.16, 17, 18, 19. The c-myc promoter is characteristic of uniquely showing both RNA polymerase II (pol II) and RNA polymerase III (pol III) activities. Previous studies demonstrated that activating PKC resulted in up-regulation of c-myc expression from its pol II promoter. PKC has recently been demonstrated to inhibit transcription from the pol III promoter of human c-myc gene20. Our present study showed that heparin, a potent inhibitor of PKC, increased the protein level of c-myc. It is possible that the overexpression of c-myc in heparin-treated CNE2 cells is related to the transcriptional inhibition of c-myc pol III promoter.
The c-myc proto-oncogene has two coupled opposing functions: proliferation and apoptosis, that suggests that other gene products may interact with c-myc so that the final output of cells could be determined. A candidate for such a modifying gene is probably bcl-221, 22, 23. Activated bcl-2 gene could prevent apoptosis induced by c-myc. The bax, another bcl-2 family gene, was observed to increase when c-myc was overexpressed24. bcl-2 can form heterodimers with bax and lose its protective effect. When bax is present in excess, cells are susceptible to programmed cell death. Thus it seems that the relative ratios of bax and bcl-2, rather than the absolute values of either genes25, determine the fate of a cell. Although we found that both bax and bcl-2 genes are expressed in cultured CNE2 cells, the changes were different in these two genes induced by heparin. The levels of bax protein in cultured CNE2 cells increased along with the increase of heparin concentrations. In contrast, the expression of bcl-2 showed no apparent changes after treatment with heparin in various concentrations. Therefore, according to the increased bax/bcl-2 ratio, the inhibitory effect of bcl-2 on c-myc-induced apoptosis might have been affected, and excessive expression of bax gene probably stimulated the apoptosis of CNE2 cells.
In conclusion, heparin induces apoptosis in CNE2 cells, which may be probably regulated by increased expression of c-myc and the rates of bax/bcl-2. These findings suggest that heparin may function as an inducer of apoptosis in carcinoma cells. Further investigation will be required to determine the spectrum of pro-apoptotic potential effects in various carcinoma cells, as well as the molecular mechanisms underlying the pro-apoptotic action of heparin in human nasopharyngeal carcinoma CNE2 cells.
Abbreviations
- PKC:
-
protein kinase C
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- NPC:
-
nasopharyngeal carcinoma
- PDGF:
-
platelet-derived growth factor
- IGF-I:
-
insulin-like growth factor-I
References
Mandal AK, Lyden TW, Saklayen MG . Heparin lowers blood pressure biological and clinical perspectives. Kidney Int 1995; 47:1017–24.
Neison RM, Cecconi O, Roberts G, Aruffo A, Linhard RJ, Bcvilaqua MP . Heparin oligosaccharides bind L-and P-selection and inhibit acute inflammation. Blood 1993; 82:3253–61.
Ley K, Cerrito M, Arfors KE . Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol 1991; 260:H1667–H1673.
Bazzoni G, Beltran-Nunez A, Mascellani G . Effect of heparin dermatan sulfate and related oligo-derivatives on human polymorphonuclear leukocyte functions. J Lab Clin Med 1993; 121:268–75.
Laghi-Pasini F, Pasqui AL, Ceccatelli L, Capecchi PL . Heparin inhibition of polymorphonuclear leukocyte action in vitro: A possible pharmacological approach to granulocyte- mediated vascular damage. Thromb Res 1984; 35:527–37.
Groggel GC, Marinides GN, Hovingh P, Hammond E . Inhibition of rat mesangial cell growth by heparin sulfate. Am J Physiol 1990; 258:F259–F265.
Krramura M, Mitaral T, Maruyama N, Nagasawa R . Mesangial cell behavior in a three-dimensional extracellular matrix. Kidney Int 1991; 40:653–61.
Gunji Y, Gorelick E . Role of fibrin coagulation in protection of murine tumor cells from destruction by cytotoxic cells. Cancer Res 1988; 48:729–36.
Markus G . The role of hemostasis and fibrinolysis in the metastatic spread of cancer. Thromb Hemost 1984; 10:61–72.
Beuth J, Ko HL, Pulverer G, Uhlenbrock G, Pichlmaier H . Importance of lectins for the prevention of bacterial infections and cancer metastasis. Glyconjugates 1995; 12:1–6.
Vlodavssky I, Ishai-Michaels R, Morhren M, Bar-Shavit R, Catani R Ekre, H-P et al. Modulation of meovascularization and metastasis by species of heparin. Adv Exp Biol Med 1992; 313:317–27.
Busch SJ, Martin GA, Barnhart RL, Jackson RL . Trans-repressor activity of nuclear glycosaminoglycans on fos and jun/AP-1 oncoprotein-mediated transcription. J Cell Biol 1992; 116:31–42.
Manaster J, Chezar J, Schurtz-Swirski R, Shapiro G, Tendler Y, Kristal B, Shasha SM, Sela S . Heparin induces apoptosis in human peripheral blood neutrophils. Br J Haematol 1996; 94:48–55.
Lshikawa Y, Kitanura M . Inhibition of glomerular cell apoptosis by heparin. Kidney Int 1999; 56:954–63.
Yang L . Yang Y-C . Heparin inhibits the expression of interleukin-11 and granulocyte-macrophage colony stimulating factor in primate bone marrow stromal fibroblasts through mRNA destabilization. Blood 1995; 86:2526–33.
Fang CM, Shi C, Xu YH . Deregulated c-myc expression in quiescent CHO cells induces target gene transcription and subsequent apoptotic phenotype. Cell Research 1999; 9:305–14.
Fan XQ, YJ Guo . Apoptosis in oncology. Cell Research 2000; 11:1–7.
Harrington E, Fanidi A, Bennett M, Evan G . Modulation of myc-induced apoptosis by specific cytokine. EMBO J 1994; 13:3286–95.
Fanidi A, Harrington E, Evan G . Cooperative interaction between c-myc and bcl-2 proti-oncogenes. Nature 1992; 359:554–6.
Xu G, Gai Q, James CB . Protein kinase C inhibits transcription from the RNA poymerase III promoter of the human c-myc gene. Cancer Lett 1998; 123:199–205.
Reed JC . Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994; 124:1–6.
Chang YC, Xu YH . Expression of Bcl-2 inhibited Fas-mediated apoptosis in human hepatocellular carcinoma BEL-7404 cells. Cell Research 2000; 10:237–42.
Yin XM . Signal transduction mediated by Bid, a pro-death Bcl-2 family. Cell Research 2000; 10:161–7.
Sakamuro D, Eviner V, Elliott KJ, Show L, White E . C-myc induces apoptosis in epithelial cell by both p53-dependent and p53-independent mechanisms. Oncogene 1995; 11:2411–8.
Oltvai ZN, Milliman CL, Korsmeyer SJ . Bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programmed cell death. Cell 1993; 74:609–19.
Acknowledgements
This work was supported by the Overseas Chinese Affairs Office of the State Council Foundation (No. 98-33) and National Natural Science Foundation of China (No. 39500056 and No. 39770300).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LI, H., YE, K., ZHANG, H. et al. Effect of heparin on apoptosis in human nasopharyngeal carcinoma CNE2 cells. Cell Res 11, 311–315 (2001). https://doi.org/10.1038/sj.cr.7290101
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290101
Keywords
This article is cited by
-
Influence of CK2 protein kinase activity on the interaction between Trypanosoma cruzi and its vertebrate and invertebrate hosts
Parasitology Research (2024)
-
Pleiotropic effects of heparins: does anticoagulant treatment increase survival in cancer patients?
Clinical and Translational Oncology (2018)
-
Ardipusilloside I induces apoptosis by regulating Bcl-2 family proteins in human mucoepidermoid carcinoma Mc3 cells
BMC Complementary and Alternative Medicine (2013)
-
Roles of vimentin and 14-3-3 zeta/delta in the inhibitory effects of heparin on PC-3M cell proliferation and B16-F10-luc-G5 cells metastasis
Acta Pharmacologica Sinica (2012)
-
Inhibitory effects of tea polyphenols by targeting cyclooxygenase-2 through regulation of nuclear factor kappa B, Akt and p53 in rat mammary tumors
Investigational New Drugs (2011)