ABSTRACT
p34cdc2 and Cyclin B1 are key components of cell cycle controlling machine and are believed to play a fundamental role in gametogenesis. It is also well known that, in scrotal mammals, spermatogenesis depends greatly on the maintenance of comparatively low temperature in the scrotum. To investigate whether the expression of cdc2 and cyclin B1 in spermatogenic cells during spermatogenesis is actually a temperature dependent event, in situ hybridization, Western blotting and immunohistochemistry analysis were used to study the expression of cdc2 and cyclin B1 in normal and cryptorchid testis. Results showed that the abdominal temperature had no significant influence on the transcription of cdc2 and cyclin B1 in the spermatogonia and pachytene/diplotene primary spermatocytes, but it blocked the translation of them. Due to the deficiency of p34cdc2 and Cyclin B1, the spermatogonia and pachytene/diplotene primary spermatocytes were unable to form MPF, hence, they couldn't undergo karyokinesis. The development of primary spermatocytes was arrested at the G2 to M phase transition. We also found that testosterone could regulate the Cyclin B1 expression in spermatogenic cells. Muscular injection of testosterone could recover spermatogenesis in the unilateral scrotal testis which was influenced by the contralateral cryptorchid testis, but it could not salvage the spermatogenesis block in the cryptorchid testis.
Similar content being viewed by others
INTRODUCTION
Temperature influences the development of germ cells and the reproductive cycle of living beings. Gametogenesis in many kinds of insects, lower vertebrate animals and hibernating animals as well as scrotal mammals depends on a suitable low- temperature condition to generate germ cells and acquires maturation competence thereafter.
Among mammals, generation of male germ cells depends mostly on the comparatively low temperature condition in scrotum. The temperature rise of testis induced by cryptorchidism, extreme heat in summer, body fever, tight clothing, sauna or other occupational high temperature conditions will cause the block or abnormality of spermatogenesis1. So far, the studies on the temperature influence on heat shock protein, heat shock factor, cold-inducible RNA binding protein, insulin-like growth factor-I receptors, ubiquitin, α-, β- and γ-DNA polymerase and topoisomerase-I as well as some other enzymes have been made. However, no conclusion is reached that these changes have direct connection with the block of spermatogenesis2. Spermatogenesis is an accurately controlled and timed process. It includes mitotic divisions of spermatogonia, meiotic divisions of spermatocytes, the differentiation and maturation of spermatids and so on. It is well known that all cell divisions are inspired by activated maturation-promoting factors (MPF) which consists of catalytic subunit p34cdc2 and regulatory subunit Cyclin B1. The activation of MPF can be achieved through sequential phosphorylation and dephosphorylation of tyrosine and threonine residues within the ATP-binding-site of p34cdc2 subunit3. It has been demonstrated that high levels of expression of cdc2 and cyclin B1 in rat and mouse testis play an important role in spermatogenesis and sperm maturation4, 5, 6, 7. So far, there has been no report on whether the spermatogenesis block induced by high temperature is related to the abnormal expression of cdc2 and cyclin B1. In this study, we studied the influence of temperature on the expression of cdc2 and cyclin B1 in rabbit spermatogenic cells and discussed the possible mechanism of spermatogenesis block by abdominal high temperature.
MATERIALS AND METHODS
Animals
Adult male New Zealand white rabbits weighted 5 kg were obtained from animal facilities of Shanghai Children's Hospital. After the rabbits were anesthetized by injecting pentobarbital sodium (24 mg/kg) via ear vein, one testis of each “test rabbit” was brought into the abdomen, sutured to the inner surface of its wall and designated as contralateral cryptorchid testis, while the other testis remained in the scrotum to act as an euthermic control, designated as unilateral scrotal testis. The “control rabbits” were performed a pseudo-operation and their testes were designated as control testes. 20 d (the duration of spermiogenesis in rabbit) later, half of the “test rabbits” were given testosterone (5 mg/day) by intra-femoral muscle injection for 38 d. On the 58th day (the average duration of spermatogenesis in a rabbit is about 2 mon8) after the surgical operation, the testes were dissected for analysis.
Preparation of slides
The testes slices were fixed at 4°C overnight in 4 % paraformaldehyde and embedded in paraffin. The paraffin embedded slices were cut into 7 μm sections which were placed onto Silane or Gelatin coated slides for in situ hybridization or immunohistochemistry analysis respectively.
In situ hybridization analysis
The pLXSN- cdc2 and pLXSN- cyclin B1 constructs were kindly provided by Dr. A. Koff9 and Dr. J. Pines10 respectively. The cdc2 and cyclin B1 cDNA were obtained from above plasmids through digestion with BamH I and EcoR I endonuclease restrictedly and then reconstructed into pSPT18 vector. Amplification, purification and identification of pSPT18- cdc2 and pSPT18- cyclin B1 plasmids were performed as described in reference11. The linearized pSPT18- cdc2 and pSPT18- cyclin B1 were obtained by digestion with EcoR I or Hind III and then transcript in vitro with T7 or SP6 RNA polymerase (DIG RNA labeling kit, Bohringer Mannheim) into sense or antisense DIG-cRNA probes, respectively12.
In situ hybridization was performed according to the descriptions of application manual12. Antisense DIG-actin cRNA probe (300 ng/ml) was used as positive control, antisense DIG- cdc2 or DIG- cyclin B1 cRNA probe (300 ng/ml) was used to detect cdc2 or cyclin B1 transcripts respectively, while sense DIG- cdc2 or DIG- cyclin B1 cRNA probe (300 ng/ml) was used as negative control. Non-probe and non anti-DIG antibody tests were also performed.
Western blotting analysis
Total proteins were extracted by homogenizing pieces of testis from “control” or “test” animals in EB (Extraction buffer: 20 mM Hepes, pH 7.5, containing 80 mM β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 200 μM PMSF, 50 mM NaF, 1 mM DTT and 3 μg/ml leupeptin), followed by ultracentrifugation (100,000 × g, 1 h, 4 °C). Proteins were separated by SDS-PAGE in 12 % gel and transferred to nitrocellulose membranes. The membranes were rinsed in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl and 0.1 % Tween-20) and blocked with block solution (5 % non-fat milk in TBST) for 30 min, followed by antibody (anti-PSTAIRE polypeptide polyclonal antibody, 5 μg IgG/ml, or anti-Cyclin B1 monoclonal antibody, 0.4 μg IgG/ml, Santa Cruz Biotechnology Inc.) incubation in block solution for 1 h. After washing (3 × 5min) with TBST, the membranes were then incubated with a 1:1,000 HRP-conjugated goat anti-mouse IgG (Sigma) in block solution for 30 min. Then the membranes were again washed (3 × 10 min) with TBST, the western blotting analysis was performed with ECL (Amersham).
Immunohistochemical analysis
The dewaxed and rehydrated sections were incubated in 10 μg/ml Saponin for 30 min, and then washed (3 × 5 min) with TBS (10 mM Tris-HCl, pH 8.0 and 150 mM NaCl,). After being blocked with block solution (1.5 % normal sheep serum in TBS) for 1 h, the specimens were incubated with antibody (anti-p34cdc2 or anti-Cyclin B1 monoclonal antibody, 2 μg IgG/ml, Santa Cruz Biotechmology) in block solution for 30 min. Having been washed (3 × 5 min) with block solution, the sections were incubated with biotin-conjugated sheep anti-mouse IgG (Vector Laboratories) in block solution for 30 min, washed with TBS for 3 × 5min, then incubated for 30 min with avidin biotin enzyme reagent, they were washed again with TBS for 3 × 5 min, rinsed in 1 % Triton X-100/TBS and in distilled water for 30 seconds respectively. Each section was color reacted and mounted as per se in the reference12.
The negative controls were performed by using normal sheep serum in substitution for primary and secondary antibody respectively.
RESULTS
Effects of temperature on spermatogenesis in rabbit testis
Spermatogonia, spermatocytes, round spermatids, elongated spermatids and spermatozoa can be found in the seminiferous epithelium of the control testis (Fig 1A), while the number of spermatids and elongated spermatids decreased in the seminiferous tubules of unilateral scrotal testis (Fig 1B). In the contralateral cryptorchid testis, there were no spermatogenic cells in all stages of seminiferous epithelium except spermatogonia and some pachytene/diplotene primary spermatocytes (Fig 1C). The interstitial tissue of the contralateral cryptorchid testis appeared to be atrophied. Intramuscular injection of testosterone could make the spermatogenesis recover in the unilateral scrotal testis (Fig 1D), but it could not improve the spermatogenesis block in the contralateral cryptorchid testis.
Effects of temperature on spermatogenesis in rabbit testis A: Control testis B: Unilateral scrotal testis C: Contralateral cryptorchid testis D: Unilateral scrotal testis after treatment with testosterone SG: Spermatogonium, SC: Spermatocyte, RS: Round Spermatid, ESm: Elongated Spermatid, Z: Spermatozoon. Black bar: 50 μm
Effects of temperature on the transcription of cyclin B1 in rabbit testis
Significant cyclin B1 transcripts could be detected in the spermatogonia and pachytene/diplotene spermatocytes of the control testis (Fig 2A) but the signal slightly decreased in spermatocytes of unilateral scrotal testis (Fig 2B). In the contralateral cryptorchid testis, we still detected the signal in the survived spermatogonia and pachytene/diplotene primary spermatocytes (Fig 2C). Treatment of testosterone could restore cyclin B1 transcription in spermatogenic cells of unilateral scrotal testis to normal level (Fig 2D), but the effect on the survived spermatogonia in contralateral cryptorchid testis was not so distinct (Fig 2E compare with 2C).
In situ hybridization analysis of the cyclin B1 transcripts in rabbit testis A: Control testis B: Unilateral scrotal testis C: Contralateral cryptorchid testis D: Unilateral scrotal testis after treatment with testosterone E: Contralateral cryptorchid testis after treatment with testosterone F: Negative control SG: Spermatogonium, SC: Spermatocyte, RS: Round Spermatid, ES: Elongated Spermatid, Black bar: 50 μm
Effects of temperature on the transcription of cdc2 in rabbit testis
In the seminiferous epithelium of control and unilateral scrotal testis, cdc2 transcripts in spermatogonia and pachytene/diplotene spermatocytes were fewer than those of cyclin B1 (Fig 3A, 3B), and mainly present in the cytoplasm. Weak signals of the cdc2 were detected in the remains of spermatogenic cells in contralateral cryptorchid testis too (Fig 3C). Testosterone treatment could improve cdc2 transcription in spermatocytes but not in spermatogonia of the unilateral scrotal testis (Fig 3D). However, the cdc2 expression was not influenced by testosterone both in spermatogonia and in spermatocytes of contralateral cryptorchid testis (Fig 3E).
In situ hybridization analysis of the cdc2 transcripts in rabbit testis A: Control testis B: Unilateral scrotal testis C: Contralateral cryptorchid testis D: Unilateral scrotal testis after treatment with testosterone E: Contralateral cryptorchid testis after treatment with testosterone F: Negative control SG: Spermatogonium, SC: Spermatocyte, RS: Round Spermatid, ES: Elongated Spermatid, Z: Spermatozoon. Black bar: 50μm
There was no positive signal detected with the cdc2 and cyclin B1 sense probe, as well as non-probe and non-anti-DIG antibody (Fig 2F, 3F).
Effects of temperature on the translation of p34 cdc2 and Cyclin B1 in rabbit testis
Western blotting analysis showed that Cyclin B1 expressed in unilateral scrotal testis (Fig 4B) was significantly lower than that of the control testis (Fig 4A), while Cyclin B1 could not be detected in contralateral cryptorchid testis (Fig 4C). After the treatment of testosterone, Cyclin B1 expression increased in unilateral scrotal testis (Fig 5B) and reached normal level as compared with control testis (Fig 5A), but still could not be detected in contralateral cryptorchid testis (Fig 5C). In the unilateral scrotal testis (Fig 6B), p34cdc2 expression was almost the same as that of the control testis (Fig 6A), but was not detected in the contralateral cryptorchid testis (Fig 6C). The injection of testosterone had little effects on p34cdc2 expression in both the unilateral scrotal (Fig 6D) and contralateral cryptorchid testis (Fig 6E).
Western blotting analysis of the effects of temperature and testosterone on the expression of p34cdc2 A: Control testis B: Unilateral scrotal testis C: Contralateral cryptorchid testis D: Unilateral scrotal testis after treatment with testosterone E: Contralateral cryptorchid testis after treatment with testosterone Each lane was loaded with 120 μg proteins
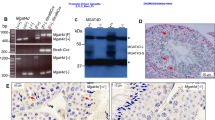
The immunohistochemical analysis results showed that Cyclin B1 existed in the spermatogenic cells of different developmental stages in the control testis and increased markedly in the round and elongated sprematids (Fig 7A). In unilateral scrotal testis, Cyclin B1 was significantly fewer than that of the control testis (Fig 7B). In contralateral cryptorchid testis, Cyclin B1 could not be detected (Fig 7C). Though testosterone treatment could induce Cyclin B1 increase in the unilateral scrotal testis (Fig 7D), it was still unable to improve Cyclin B1 expression in contralateral cryptorchid testis (Fig 7E). p34cdc2 was expressed mainly in the pachytene/ diplotene primary spermatocyte of control testis (Fig 8A). The expression of p34cdc2 in the unilateral scrotal testis was slightly weaker than that of the control testis (Fig 8B) while very few of p34cdc2 could only be detected in some spermatogonia of the contralateral cryptorchid testis (Fig 8C). Testosterone injection could ameliorate p34cdc2 expression in unilateral scrotal (Fig 8D) but no in contralateral cryptorchid testis (Fig 8E). The results above were accordant with those of Western blotting. We did not observe any significant immunostaining in the negative control groups (Fig 7F, 8F).
Immunohistochemical analysis of the effects of temperature and testosterone on the expression of Cyclin B1 A: Control testis B: Unilateral scrotal testis C: Contralateral cryptorchid testis D: Unilateral scrotal testis after treatment with testosterone E: Contralateral cryptorchid testis after treatment with testosterone F: Negative control SG: Spermatogonium, SC: Spermatocyte, RS: Round Spermatid, Black bar: 100 μm
Immunohistochemical analysis of the effects of temperature and testosterone on the expression of p34cdc2 A: Control testis B: Unilateral scrotal testis C: Contralateral cryptorchid testis D: Unilateral scrotal testis after treatment with testosterone E: Contralateral cryptorchid testis after treatment with testosterone F: Negative control SG: Spermatogonium, SC: Spermatocyte, RS: Round Spermatid, Black bar: 50 μm
DISCUSSION
Temperature higher than that in scrotum leading to the blocking of the transition of male germ cell from mitosis to meiosis
The spermatogenesis of scrotal mammals depends on the comparatively low temperature condition in scrotum. Temperature higher than the scrotal temperature (e. g. abdominal temperature) would block the spermatogenesis. Hence, the scrotal mammals were often chosen as the model to study the influences of temperature on the structures and functions of spermatogenic or other cells in testis2. In general, spermatogenic and other cells of testis were sensitive to heat, and if testis is submitted even to the so called “normal body temperature” (such as 37°C in humans) for a long time, not only spermatogenesis will be blocked, but also Leydig and Sertoli cells will be hurt to a great extent2, 13. Cytological studies showed that heat would differentially hurt male germ cells in different developmental stages during spermatogenesis, especially the pachytene primary spermatocytes2, 13.
According to our results, most of spermatogonia in contralateral cryptorchid testis were not harmed fatally by heat as yet, indicating that spermatogonia could resist to heat to a certain extent, even though the mechanism involved is not clear so far. In this case spermatogonia could develop to pachytene/diplotene primary spermatocytes, but they could not acquire the ability to complete the transition from mitosis to meiosis, and then appeared to go through apoptosis. Therefore, we could not find the descendants of meiosis: secondary spermatocytes and round spermatids, elongated spermatids and spermatozoon. It is well known, MPF is the major driver of cell division and all factors that interfering with the formation and activation of MPF will block cell division. We therefore analyzed the transcription and translation of each subunit of MPF (p34cdc2 and Cyclin B1) to reveal the possible underlying mechanism of heat block of spermatogenesis.
The results of in situ hybridization showed that cyclin B1 transcripts were mainly expressed in the spermatogonia and pachytene/diplotene primary spermatocytes of control testis. With the advance of cell division, the hybridization signal decreased gradually from spermatocytes to round spermatids. When the spermatids become elongated, cyclin B1 transcripts were disappeared. cdc2 was only transcribed in the spermatogonia and pachytene/diplotene primary spermatocytes before meiosis. In the contralateral cryptorchid testis, the cyclin B1 and cdc2 transcripts still could be detected in spermatogonia and pachytene/diplotene primary spermatocytes, which means that high temperature did not considerably block the transcription of cyclin B1 and cdc2 in these cells.
The results of immunohistochemistry displayed that p34cdc2 was expressed mainly in both spermatogonia and pachytene/diplotene primary spermatocytes. Cyclin B1 increased in the pachytene/diplotene primary spermatocytes before meiosis and reached its peak in the spermatids. Obviously, large amounts of Cyclin B1 existing in spermatids has no effect on adjusting the activity of p34cdc2 kinase, which may be just a signal of the end of cell division and/or a necessary factor for the initiation of the metamorphosis of spermatids4. On the contrary, Cyclin B1 could not be detected in all cell types of the contralateral cryptorchid testis, even though p34cdc2 could be detected in some spermatogonia, a fact which could be referred to the inhibitory effect of high temperature on periodic biosynthesis of Cyclin B1 and p34cdc214.
These results showed that abdominal high temperature did not affect seriously the transcription of cyclin B1 and cdc2, but inhibit the translation of Cyclin B1 and p34cdc2 in the spermatogonia and pachytene/diplotene primary spermatocytes. Due to the deficiency of Cyclin B1 and p34cdc2, spermatogonia and pachytene/diplotene primary spermatocytes in contralateral cryptorchid testis were unable to form MPF, and therefore, they could not accomplish their nuclear division, and became arrested at the transition stage from mitosis to meiosis (G2/M phase).
Testosterone is capable of adjusting the Cyclin B1 expression at translation level
Our results showed that the spermatogenesis in unilateral scrotal testis was influenced by the contralateral cryptorchid testis. In some seminiferous tubules of unilateral scrotal testis, the number of round spermatids and metamorphosing spermatids decreased significantly and, in some of them even could not be observed. In the meantime, the expression of Cyclin B1 was also decreased. To examine the possible mechanism of these changes, half of “test rabbits” were treated with testosterone. Although testosterone injection could not improve blocked spermatogenesis in contralateral cryptorchid testis, it could recover spermatogenesis and increase the Cyclin B1 expression to almost the normal level in unilateral scrotal testis. These results indicated that testosterone could regulate Cyclin B1 expression in spermatogenic cells of unilateral scrotal testis. It has been reported that the metamorphosis of round spermatids depended on testosterone during spermatogenesis, but the mechanism was not mentioned15. As cryptochidism could cause the decrease of testosterone level in many kind of animals16, 17, 18, we believe that the decrease of Cyclin B1 expression due to the shortage of testosterone supply may be the main reason for the decrease and deficiency of round spermatids and other metamorphosing spermatids in unilateral scrotal testis. Therefore, we conclude that the spermatogenesis in scrotal mammals need to proceed not only in a comparatively low temperature condition, but also in a condition with adequate supply of testosterone to maintain the normal spermatids, especially their metamorphosis.
High temperature inducing the deletion of MPF and the apoptosis of spermatogenic cells
Abdominal temperature will increase the risk of apoptosis in spermatogenic cells, but its mechanism is not clear18, 19, 20. Though it is reported that p53 plays an important role in heat induced spermatogenic cell apoptosis21, 22, but apoptosis also appears in the mouse cryptorchid testis which lacks p5322. So it is possible that the expression of p53 may not be the direct reason for heat induced spermatogenic cell apoptosis and spermatogenetic block22, 23. Eddy reported that if Cyclin B1 could not combine with p34cdc2 to form heterodimer, the spermatogenesis would be blocked at the G2/M transition of the first meiosis, then all pachytene primary spermatocytes would go through apoptosis, leading finally to spermatids depletion24. Taken together, the above data lead us to infer that MPF is one of the most important factors that not only can promote cell division, but also prevent excessive apoptosis in spermatogenic cells during spermatogenesis.
The possible activity of Y-box binding protein in spermatogenesis
It is well known that low temperature can induce and impel the synthesis of some proteins. Among them, cold-shock protein is the most conspicuous. Y-box (inverted CCAAT box) binding proteins are special members of cold-shock protein family, which can bind DNA as well as mRNA to alter both transcription and translation of cells25. Like RNA chaperon, the conserved sequence of Y-box binding proteins destabilizes the RNA secondary structure and regulates cell translation in low temperature26. As Y-box binding proteins exist widely in the spermatocytes of scrotal mammals27, we believe that they may have important effects on the inverted CCAAT box of cdc2 gene promoter, and then control the transcription of p34cdc2. Meanwhile, they can also combine with cyclin B1 mRNA and play significant roles in cell cycle28. Recent report showed that a cold-shock protein, Rbm3, was decrease in Sertoli cells when mouse testis was exposed to heat stress by experimental cryptorchidism29. Thus, we would like to speculate that the further studies on the cold-shock proteins of spermatogenic cells at the scrotal low temperature may give insight to uncover the functions of cold- shock proteins in relation to spermatogensis, including the control mechanism of gene transcription and translation in heat induced spermatogenetic block.
References
Thonneau P, Bujan L, Multigner L, Mieusset R . Occupational heat exposure and male fertility: a rewiew. Hum Reprod 1998; 13:2122–5.
Setchell BP . The parkes lecture. Heat and the testis. J Reprod Fertil 1998; 114:179–94.
Meijer, L, Azzi L, Wang JY . Cyclin B targets p34cdc2 for tyrosine phosphorylation. EMBO J 1991; 10:1545–54.
Chapman DL, Wolgemuth DJ . Identification of a mouse B-type cyclin which exhibits developmentally regulated expression in the germ line. Mol Reprod Dev 1992; 33:259–69.
Gromoll J, Wessels J, Rosiepen G, Brinkworth MH, Weinbauer GF . Expression of mitotic cyclin B1 is not confined to proliferation cells in the rat testis. Biol Reprod 1997; 57:1312–9.
Rhee K, Wolgemuth DJ . CDK family genes are expressed not only in dividing but also in terminally differentiated mouse germ cells, suggestion their possible function during both cell division and differentiation. Dev Dyn 1995; 204:406–20.
Chapman DL, Wolgemuth DJ . Regulation of M-phase promoting factor activity during development of mouse male germ cells. Dev Biol 1994; 165:500–6.
Courot MT, de Reviers H, Ortavant R . Spermatogenesis. In: Johnson AD, Gomes WR, Vandemark NL. Eds. The testis: development, anatomy and physiology. Academic press: New York and London, 1970:339–411.
Koff A, Cross F, Fisher A . et al. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell 1991; 66:1217–28.
Pines J, Hunter T . Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2}. Cell 1989; 58:833–46.
Sambrook J, Fritsch EF, Maniatis T . eds. Molecular cloning, second edition. Cold Spring Harbor Laboratory Press, 1989.
Grunewald-Janho S, Keesey J, Leous M, van Miltenburg R, Schroeder C . eds. Nonradioactive in situ hybridization application manual, second edition. Boehringer Mannheim GmbH Press, 1996.
Waites GMH and Setchell BP . Lamming GE . eds. Marshall's Physiology of Reproduction. Charchill Livingstone Press, 1990.
McGowan CH, Russell P, Reed S . Periodic biosynthesis of the human M-phase promoting factor catalytic subunit p34 during the cell cycle. Mol Cell Biol 1990; 10:3847–51.
O'Donnell L, McLachlan RI, Wreford NG, Robertson DM . Testosterone promotes the conversion of round spermatids between stages 7 and 8 of the rat spermatogenic cycle. Endocrinology 1994; 135:2608–14.
Ganjam VK, Kenney RM . Androgens and oestrogens in normal and cryptorchid stallions. J Reprod Fertil Suppl 1975; 23:67–73.
Sharp RM, Kerr JB, Fraser HM, Bartlett JM . Intratesticular factors and testosterone secretion. Effect of treatments that alter the level of testosterone within the testis. J Androl 1986; 7:180–9.
Ohta Y, Nishikawa A, Fukazawa Y, Urushitani H, Matsuzawa A, Nishina Yihuchi T . Apoptosis in adult mouse testis induced by experimental cryptorchidism. Acta Anat 1996; 157:195–204.
Shikone T, Billig H, Hsueh AJ . Experimentally induced cryptorchidism increases apoptosis in rat testis. Biol Reprod 1994; 51:865–72.
Yin Y, Hawkins KL, DeWolf WC, Morgentaler A . Heat stress causes testicular germ cell apoptosis in adult mice. J Androl 1997; 18:159–65.
Socher SA, Yin Y, DeWolf WC, Morgentaler A . Temperature-mediated germ cell loss in the testis is associated with altered expression of the cell-cycle regulator p53. J Urol 1997; 157:1986–9.
Yin Y, DeWolf WC, Morgentaler A . Experimental cryptorchidism induces testicular germ cell apoptosis by p53-dependent and -independent pathways in mice. Biol Reprod 1998; 58:492–6.
Morgentaler A, Stahl BC, Yin Y . Testis and temperature: an historical, clinical, and research perspective. J Androl 1999; 20:189–95.
Eddy E.M . Hsp70-2 heat shock protein of mouse spermatogenic cells. J Exp Zool 1998; 282:261–71.
Matsumoto K, Wolffe AP . Gene regulation by Y-box proteins: coupling control of transcription and translation. Trends Cell Biol 1998; 8:318–23.
Jiang W, Hou Y, Inouye M . CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperon. J Biol Chem 1997; 272:196–202.
Gu W, Tekur S, Reinbold R, Eppig JJ, Choi YC, Zheng JZ, Murray MT, Hecht NB . Mammalian male and female germ cells express a germ cell-specific Y-box protein, MSY2. Biol Reprod 1998; 59:1266–74.
Katsu Y, Yamashita M, Nagahama Y . Isolation and characterization of goldfish Y-box protein, a germ- cell- specific RNA-binding protein. Eur J Biochem 1997; 249:854–61.
Danno S, Itoh K, Matsuda T, Fujita J . Decreased expression of mouse Rbm3, a cold-shock protein, in Sertoli cells of cryptochid testis. Am J Pathol 2000; 156:1685–92.
Acknowledgements
This project was supported by National Natural Science Foundation of China. Grant Nos: 39770370, 39630160. We are grateful to Dr. A. Koff and Dr. J. Pines for pLXSN- cdc2 and pLXSN- cyclin B1 constructs respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
KONG, W., GU, Z., LU, J. et al. Temperature dependent expression of cdc2 and cyclin B1 in spermatogenic cells during spermatogenesis. Cell Res 10, 289–302 (2000). https://doi.org/10.1038/sj.cr.7290056
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290056
Keywords
This article is cited by
-
Photobiomodulation therapy reverses spermatogenesis arrest in hyperthermia-induced azoospermia mouse model
Lasers in Medical Science (2023)
-
Testis-enriched heat shock protein A2 (HSPA2): Adaptive advantages of the birds with internal testes over the mammals with testicular descent
Scientific Reports (2016)
-
Knockdown of CDC2 expression inhibits proliferation, enhances apoptosis, and increases chemosensitivity to temozolomide in glioblastoma cells
Medical Oncology (2015)
-
Heat stress response of male germ cells
Cellular and Molecular Life Sciences (2013)
-
The inhibition of lung cancer cell growth by intracellular immunization with LC-1 ScFv
Cell Research (2002)