Abstract
Background:
Small quantities of normal saline are sometimes instilled into the endotracheal tube of intubated neonates, to assist with the removal of thick secretions and maintain patency of the endotracheal tube. However, saline is detrimental to the innate immune system of the upper airway mucosa, rapidly unfolding and inactivating antimicrobial peptides such as LL-37. We previously reported the preparation and feasibility testing of ‘ETCare’, a low-sodium, physiologically based solution for airway care, and we now report results of a randomized, masked, controlled, two-centered study testing ETCare vs sterile saline among 60 intubated NICU patients.
Study design:
Sixty intubated NICU patients were randomized to having their airway care with ETCare vs saline. Three hypotheses were tested: (1) tolerance – patients will tolerate ETCare for airway care as well as they tolerate saline, (2) nosocomial infections – ETCare will result in fewer tracheal aspirates where organisms grow and fewer cases of nosocomial sepsis, and (3) chronic lung disuse – ETCare will result in fewer patients discharged home on supplemental O2.
Results:
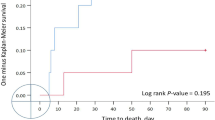
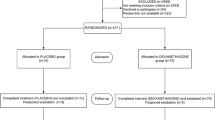
Thirty NICU patients with an endotracheal tube in place were randomized to receive their airway care with ETCare, and 30 to receive their care with saline. Only the pharmacist was aware of the randomization; the two solutions were visually indistinguishable and were dispensed in identical syringes. Tolerance of the solutions was similar. The ETCare recipients had trends toward fewer positive blood cultures (odds ratios (OR), 0.48; 95% confidence interval (CI), 0.13 to 1.68), and fewer discharges home on supplemental O2 (OR, 0.43; 95% CI, 0.14 to 1.32; P=0.075).
Conclusions:
On the basis of this study and our previous 10-patient feasibility trial, we maintain that, for airway care, intubated NICU patients tolerate ETCare as well as saline. Data from this study can be used in estimating the sample sizes needed for a phase III trial. We speculate that such a trial will demonstrate that, compared with saline, ETCare will result in fewer nosocomial infections and less chronic lung disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hagler DA, Traver GA . Endotracheal saline and suction catheters: sources of lower airway contamination. Am J Crit Care 1994; 3: 444–447.
Trawoger R, Kolobow T, Cereda M, Giacomini M, Usuki J, Horiba K et al. Clearance of mucus from endotracheal tubes during intratracheal pulmonary ventilation. Anesthesiology 1997; 86: 1367–1374.
Kinloch D . Instillation of normal saline during endotracheal suctioning: effects on mixed venous oxygen saturation. Am J Crit Care 1999; 8: 231–240.
Lindgren S, Almgren B, Hogman M, Lethvall S, Houltz E, Lundin S et al. Effectiveness and side effects of closed and open suctioning: an experimental evaluation. Intens Care Med 2004; 30: 1630–1637.
Cunha-Goncalves D, Perez-de-Sa V, Ingimarsson J, Werner O, Larsson A . Inflation lung mechanics deteriorates markedly after saline instillation and open endotracheal suctioning in mechanically ventilated healthy piglets. Pediatr Pulmonol 2007; 42: 10–14.
Schulze A . Respiratory gas conditioning in infants with an artificial airway. Semin Neonatol 2002; 7: 369–377.
Zabner J, Sieler MP, Launspach JL, Karp PH, Kearney WR, Look DC et al. The osmolyte xylitol reduces the salt concentration of airway surface liquid and may enhance bacterial killing. Proc Natl Acad Sci USA 2000; 97: 11614–11619.
Zabner J, Smith JJ, Karp PH, Widdicombe JH, Welsh MJ . Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol Cell 1998; 2: 397–403.
Smith JJ, Travis SM, Greenberg EP, Welsh MJ . Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 1996; 85: 229–236.
Singh PK, Tack BF, McCray PB, Welsh M . Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol 2000; 279: L799–L805.
Travis SM, Conway BA, Zaabner J, Smith JJ, Anderson NN, Singh PK et al. Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol 1999; 20: 872–879.
Bals R . Epithelia antimicrobial peptides in host defense against infection. Current Sci Ltd Resp Res 2000; 1: 141–150.
Ganz T . Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest 2002; 109: 693–697.
Bals R, Wilson JM . Cathelicidins – a family of multifunctional antimicrobial peptides. Cell Mol Life Sci 2003; 60: 711–720.
Wang Y, Walter G, Herting E, Agerberth B, Johansson J . Antibacterial activities of the cathelicidins prophenin (residues 62 to 79) and LL-37 in the presence of a lung surfactant preparation. Antimicob Agents Chemother 2004; 48: 2097–2100.
Putsep K, Carlsson G, Boman HG, Anderson M . Deficiency of antibacterial peptides in patients with morbus Kostmann: an observational study. Lancet 2002; 360: 1144–1149.
Cirioni O, Giacometti A, Chiselli R, Bergnach C, Orlando F, Silvestri C et al. LL-37 protects rats against lethal sepsis caused by Gram-negative bacteria. Antimicrob Agents Chemother 2006; 50: 1672–1679.
Woodhead DD, Lambert DK, Scoffield S, Snow G, Baer V, Christensen RD . Feasibility and preliminary safety study of a physiologically based solution for airway care in the neonatal intensive care unit. Neonatal Intensive Care 2006; 19: 29–33.
Jimenez-Reyes M, Sanchez-Aguirre FJ . Sodium and chlorine concentrations in mixed saliva of healthy and cystic fibrosis children. Appl Radiat Isot 1996; 47: 273–277.
Ben-Aryeh H, Lapid S, Szargel R, Benderly A, Gutman G . Composition of whole unstimulated saliva of human infants. Arch Oral Biol 1984; 29: 357–363.
Fanaroff AA, Korones SB, Wright LL, Verter J, Poland RL, Bauer CR et al. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J 1998; 17: 593–598.
Devlin LA, Lassiter HA . Immunoenhancement to prevent nosocomial coagulase-negative staphylococcal sepsis in very low-birth-weight infants. Clin Perinatol 2004; 31: 69–75.
Kamagata-Kiyoura Y, Abe S, Yamaguchi H, Nitta T . Protective effects of human saliva on experimental murine oral candidiasis. J Infect Chemother 2004; 10: 253–255.
Borderon JC, Therizol-Ferly M, Saliba E, Laugier J, Quentin R . Prevention of Candida colonization prevents infection in a neonatal unit. Biol Neonate 2003; 84: 37–40.
Austin NC, Darlow B . Prophylactic oral antifungal agents to prevent systemic candida infection in preterm infants. Cochrane Database Syst Rev 2004 CD003478.
Hong IP, Lee SJ, Kim YS, Choi SG . Recombinant expression of human cathelicidin (hCAP18/LL-37) in Pichia pastoris. Biotechnol Lett 2007; 29: 73–78.
Moon JY, Henzler-Wildman KA, Ramamoorthy A . Expression and purification of a recombinant LL-37 from Escherichia coli. Biochim Biophys Acta 2006; 1758: 1351–1358.
Acknowledgements
This study was supported in part by a grant from the Deseret Foundation, Salt Lake City, Utah.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website (http://www.nature.com/jp)
Supplementary information
Rights and permissions
About this article
Cite this article
Christensen, R., Rigby, G., Schmutz, N. et al. ETCare: a randomized, controlled, masked trial comparing two solutions for upper airway care in the NICU. J Perinatol 27, 479–484 (2007). https://doi.org/10.1038/sj.jp.7211779
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211779