Abstract
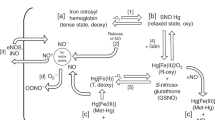
Nitric oxide (NO) is a gas that has potent vasodilator properties. It can be administered via inhalation in situations where NO production is impaired and results in vasodilatation of the pulmonary capillaries. In term infants, the administration of inhaled NO, at a dose of 20 parts per million, may reduce the need for extracorporeal membrane oxygenation by reducing pulmonary vascular resistance and improving oxygenation. Inhaled NO is an approved therapy in term babies with severe hypoxemic respiratory failure. In premature infants, inhaled NO may increase bleeding time and decrease platelet aggregation resulting in an increased risk for intraventricular hemorrhage. Early administration of inhaled NO may also potentially decrease the risk for developing chronic lung disease in premature infants. However, since studies show conflicting results, inhaled NO should only be used in premature neonates following investigational review board approved protocols.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Furchgott RF, Zawadzki JV . The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980; 288: 373–376.
Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G . Endothelium-derived relaxing factor produced and released from artery and vein in nitric oxide. Proc Natl Acad Sci USA 1987; 84: 9265–9269.
Palmer RM, Ferrige AG, Moncada S . Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987; 327: 524–526.
Murad F . Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest 1986; 78: 1–5.
Yoshida K, Kasama K . Biotransformation of nitric oxide. Environ Health Perspect 1987; 73: 201–206.
Ignarro LJ . Biological actions and properties of endothelium derived nitric oxide formed and released from artery and vein. Circ Res 1989; 65: 1–17.
Kinsella JP, Abman SH . Recent developments in the pathophysiology and treatment of persistent pulmonary hypertension of the newborn. J Pediatr 1995; 126: 853–864.
Kinsella JP, Neish SR, Shaffer E, Abman SH . Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992; 340: 819–820.
Levin DL, Heymann MA, Kitterman JA, Gregory GA, Phibbs RH, Rudolph AM . Persistent pulmonary hypertension of the newborn. J Pediatr 1976; 89: 626–630.
Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA et al. Low dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 2000; 342: 469–474.
Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose response, multicenter study. The INO/PPHN Study group. Pediatrics 1998; 101: 325–334.
Roberts Jr JD, Fineman JR, Morin III FC, Shaul PW, Rimar S, Schreiber MD et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med 1997; 336: 605–610.
Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full term and nearly full- term infants with hypoxic respiratory failure. N Engl J Med 1997; 336: 597–604.
Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R et al. Safety of withdrawing inhaled nitric oxide therapy in persistent pulmonary hypertension of the newborn. Pediatrics 1999; 104: 231–236.
Abman SH, Kinsella JP, Parker TP, Storme L, Cras TD . Physiologic roles of nitric oxide in the prenatal pulmonary circulation. In: Weir EK, Archer SL, Reeves JT (eds). Fetal and Neonatal Pulmonary Circulations. Futura: New York, 1999 pp 239–260.
Parker TA, Le Cras TD, Kinsella JP, Abman SH . Developmental changes in endothelial nitric oxide synthase expression in the ovine fetal lung. Am J Physiol 2000; 278: L202–L208.
Shaul PW, Afshar S, Gibson LL, Sherman TS, Kerecman JD, Grubb PH et al. Developmental changes in nitric oxide synthase isoform expression and nitric oxide production in fetal baboon lung. Am J Physiol Lung Cell Mol Physiol 2002; 283: L1192–L1199.
Hogman M, Frostell C, Arnberg H, Hedenstierna G . Bleeding time prolongation and nitric oxide inhalation. Lancet 1993; 341: 1664–1665.
Cheung PY, Salas E, Etches PC, Phillipos E, Schulz R, Radomski MW . Inhaled nitric oxide and inhibition of platelet aggregation in critically ill neonates. Lancet 1998; 351: 1181–1182.
Van Meurs KP, Rhine WD, Asselin JM, Durand DJ, the preemie NO Collaborative Group. Response of premature infants with severe respiratory failure to inhaled nitric oxide. Pediatr Pulmonol 1997; 24: 319–323.
Kinsella JP, Walsh WF, Bose C, Gerstmann DR, Labella JJ, Sardesai S et al. Randomized control trial of inhaled nitric oxide in premature neonates with respiratory failure. Lancet 1999; 354: 1066–1071.
Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, Abman SH . Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res 1997; 41: 457–463.
Hamon I, Fresson J, Nicolas MB, Buchweiller MC, Franck P, Hascoet JM . Early nitric oxide improves oxidative balance in very preterm infants. Pediatr Res 2005; 57: 637–643.
Schreiber MD, Gin-Mestran K, Marks JD, Huo D, Lee G, Srisuparp P . Inhaled nitric oxide in premature infants with respiratory distress syndrome. N Engl J Med 2003; 349: 2099–2107.
Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med 2005; 353: 13–22.
Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD . Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. N Engl J Med 2005; 353: 23–32.
Subhedar NV, Shaw NJ . Changes in oxygenation and pulmonary haemodynamics in preterm infants treated with inhaled nitric oxide. Arch Dis Child 1997; 77: F185–F190.
The Franco-Belgium Colloborative Trial Group. Early compared with delayed inhaled nitric oxide in moderately hypoxemic neonates with respiratory failure; a randomized controlled trial. Lancet 1999; 354: 1066–1071.
Hascoet JM, Fresson J, Claris O, Hamon I, Lombet J, Liska A et al. The safety and efficacy of nitric oxide therapy in premature infants. J Pediatr 2005; 146: 318–323.
Field D, Elbourne D, Truesdale A, Grieve R, Hardy P, Fenton AC et al. Neonatal ventilation with inhaled nitric oxide versus ventilatory support without inhaled nitric oxide for preterm infants with severe respiratory failure: the INNOVO multicentre randomized controlled trial (15RCTN 17821339). Pediatrics 2005; 115: 926–936.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: The author is on the Speaker's Bureau for INO Therapeutics Inc.
Rights and permissions
About this article
Cite this article
Sekar, K. Inhaled nitric oxide in term and preterm infants. J Perinatol 26 (Suppl 1), S4–S7 (2006). https://doi.org/10.1038/sj.jp.7211497
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211497