Abstract
Small populations of our study species Ranunculus reptans have reduced fitness because of inbreeding, genetic load, and reduced mate availability; that is, they suffer from a three-fold genetic Allee effect. Here, we investigate how the effect of interpopulation outbreeding on offspring fitness depends on population size. We performed within- and between-population crosses with plants originating from 15 populations, and measured offspring performance in a common environment. Interpopulation outbreeding led to an increase in population means of clonal performance, which was defined as the number of rooted offspring rosettes produced per maternal ovule. This fitness gain mainly occurred at the life stage of seed set. It was especially pronounced for populations with a long-term history of small size inferred from their low genetic diversity, estimated from eight allozyme loci. We conclude that in a self-incompatible plant such as R. reptans, interpopulation outbreeding can lead to an immediate genetic rescue effect due to increased cross-compatibility and heterosis, and that this rescue effect is increased as population size decreases.
Similar content being viewed by others
Introduction
Numerous studies of plants (and animals) have recorded reduced fitness in small populations in nature and when individuals from small populations are raised in a common environment (Menges, 1991; Oostermeijer et al, 1994; Heschel and Paige, 1995; Fischer and Matthies, 1998; Morgan, 1999; Fischer et al, 2000a; Kéry et al, 2000; Luijten et al, 2000; Schmidt and Jensen, 2000; Mavraganis and Eckert, 2001; Jacquemyn et al, 2002; Paschke et al, 2002; Severns, 2003; Vergeer et al, 2003; Brys et al, 2004). There are three likely genetic causes for reduced fitness of plants from smaller populations. The expression of inbreeding depression is more pronounced in small populations because of increased levels of inbreeding (Keller and Waller, 2002). Increased genetic load through drift in small populations is predicted by theory (Wright, 1931; Kimura et al, 1963; Lynch et al, 1995) and was recently affirmed in two studies of natural plant populations (Paland and Schmid, 2003; Willi et al, 2005). Reduced seed set in small populations of self-incompatible species has been recorded in several studies (Widén, 1993; Luijten et al, 2000; Fischer et al, 2003), probably because of a lack of compatible partners.
Interpopulation gene flow may counteract some of these processes. Outbreeding can lead to an increase in heterozygosity, which is beneficial if recessive deleterious alleles are masked or if heterozygosity is of a general fitness advantage (Wright, 1977). Theory predicts heterosis in crosses between individuals from populations that are connected by some level of migration. Whitlock et al (2000) showed that heterosis is expected to be highest when the effective population size is small (<1000 individuals), mutation rates and selection coefficients are intermediate, migration rates are low, and when alleles are recessive. In self-incompatible plants, outbreeding may also enrich the pool of S-alleles and increase mate availability, suggesting a further fitness increase.
A few plant studies have measured the fitness consequences of intra- and interpopulation crosses in the context of population size (Van Treuren et al, 1993; Hauser and Loeschcke, 1994; Ouborg and Van Treuren, 1994; Heschel and Paige, 1995; Paland and Schmid, 2003). Only two of these included a sufficient number of populations (>5) to estimate effects of population size (Van Treuren et al, 1993; Paland and Schmid, 2003). They found moderate to substantial fitness increases from interpopulation outbreeding, but not a consistent pattern of small populations benefiting most. This may result from their estimates of population size from current census sizes, which need not reflect long-term population sizes.
We investigated whether fitness consequences of interpopulation outbreeding depended on population size in the self-incompatible plant Ranunculus reptans. Small populations of this species have been recorded to suffer from a lack of compatible breeding partners, inbreeding depression, and increased fixed drift load (Willi et al, 2005). We studied 13 populations from Lake Constance, assuming that they had experienced similar population histories and mainly differed in size. As a measure of long-term population size, we used allelic diversity, which is correlated with current population size in the field, but is uncorrelated with population isolation (Y Willi, unpublished data). We focused on the following questions: (1) Do populations benefit from interpopulation outbreeding? (2) Is this fitness gain higher in smaller populations?
Methods
Species
R. reptans (Ranunculaceae) has a circumpolar distribution, mainly in the temperate to boreal-subarctic zones of Europe, Asia and North America (Prati and Peintinger, 2000). The species reaches its southern limit in central Europe, where this study was conducted. Here, R. reptans usually occurs in relatively distinct populations at the shores of prealpine lakes. The populations are probably relicts of the last ice age (Prati and Peintinger, 2000). Apart from the natural scarcity of the appropriate habitat, the destruction of natural shorelines during the last century has created further habitat fragmentation.
R. reptans is self-incompatible (Prati and Peintinger, 2000). Studies of the genetic basis of incompatibility in the genus Ranunculus suggest that the species possesses a gametophytic self-incompatibility system (Lundqvist, 1990, 1994; De Nettancourt, 2001). Self-incompatibility is maintained even in populations of very small size (Y Willi, unpublished data). The plant grows clonally and nodes may develop roots. Only rooted rosettes survive the annual floods caused by snow melt, and the plants grow only clonally and do not produce flowers when under water. In many years the period remaining after summer inundation is too short to allow successful seed production and seedling establishment, which makes clonal reproduction an especially important fitness component in this species.
Sampling
We used 187 plants that had been collected from 13 R. reptans populations around Lake Constance, one population near the Bernina Pass, and one at Lago Maggiore, in Switzerland in spring 2002. At each site, 14 individuals were collected at 5 m intervals along two transects separated by 5 m. The distance between the two transects was decreased to 4 m in four narrow populations. In six populations, the band of R. reptans along the shoreline was so restricted that we could only sample 8–12 individuals. The number of sampled individuals was not correlated with population size (P>0.2, N=13 populations from Lake Constance, which served as target populations). After collection, plants were maintained in a growth room until the end of the crossing experiment. Five original plants died during the propagation phase.
Crossing design
All 13 Lake Constance populations were used as target populations, for which effects of within- and between-population crossing were studied. Plants of each target population were crossed with plants of two other populations following a near–far design. The ‘near’ partner population came from the same basin of Lake Constance, and these shared relatively high genetic similarity (Fischer et al, 2000b). The ‘far’ population came from a different lake basin or a different region. In the two cases, the ‘near’ population came from a separate lake basin, but RAPD-based ΦST and allozyme-based FST were both low (< 0.04; Fischer et al, 2000b; Y Willi, unpublished data). Populations were used as partners once or twice, with the exception of the Lago Maggiore population, which was used three times.
We crossed all genotypes from the 13 Lake Constance populations with two other genotypes from the same population, along with one genotype from each of the two partner populations. This procedure resulted in a total of 664 cross combinations, each of which was performed reciprocally (each flower served as a pollen donor and recipient). Crossings took place over a period of 2 months, from 29 September to 27 November 2002, with eight later crosses among late blooming genotypes. We harvested seeds (nutlets) about 1 month after crossing, and counted the number of developed seeds and ovules. Some reciprocal crosses produced no seeds and were therefore recognized as imcompatible.
Measurements of offspring fitness
We measured sexual and clonal components of fitness in adult plants of the F1 generation. In May 2003, to start the F1 generation, seed families were incubated in gibberellic acid (2 g in 1 l of water) for 5 days before germinating indoors (16 h daylight) on a 3:1 mixture of horticulture soil and sand. The positions of the trays were rerandomized at weekly intervals. Six weeks after germination began (16–18 June 2003), we counted seedlings and haphazardly chose one per seed family for planting into a tub (10 × 10 × 11 cm3) with a 1:2 mixture of horticultural soil and sand. We distributed tubs in random positions within outdoor beds covered with 50% shade cloth. The plants were watered daily, unless it rained. We monitored plant survival after 3 days, 2 weeks, and 4 weeks. A total of 19 plants of within-population crosses and 18 of between-population crosses died. We replaced these plants by another representative of the same seed family. Tub positions were rerandomized after 1 month, and after 2 months we assessed both clonal and sexual reproduction. We counted and measured the following parameters: number of rooted rosettes, number of flowers, number of flower buds, number of infructescenses, fresh biomass (after drying plants with paper towels), and average proportion of developed seeds per infructescense.
For each cross, offspring fitness was assessed individually for the sexual and clonal performance of its progeny. Sexual performance was the proportion of maternal ovules that produced seedlings (seedling emergence) multiplied by the total number of flowers and buds produced by the family representative and by a factor representing seed production ( × 1 or × 2, for 0–50% seed production, or >50%, respectively). Our estimate of clonal performance was seedling emergence multiplied by the number of rooted rosettes of the family representative.
Allozyme electrophoresis and population size
Plants were scored at eight loci of seven enzyme systems: AAT-1 (EC 2.6.1.1), ACON-1 (EC 4.2.1.3), GPI-2 (EC 5.3.1.9), MDH-2 (EC 1.1.1.37), MDH-3, MPI (EC 5.3.1.8), SKD (EC 1.1.1.25), and 6-PGDH (EC 1.1.1.44) according to standard methods (Hebert and Beaton, 1993). Allelic diversity (Hs) was calculated as a measure of genetic variation (Nei, 1973). Hs represents long-term population size under the assumption of mutation-drift balance (Frankham, 1996), and was positively correlated with R. reptans population sizes as measured in the field (Willi et al, 2005). Hence, we used allelic diversity as a surrogate for long-term population size. The software program SPAGeDi (Hardy and Vekemans, 2002) was used to calculate pairwise FST values between populations.
Statistical analysis
To analyze fitness consequences of interpopulation outbreeding in populations of varying size, we calculated means of within- and between-population crosses for each target population. Each cross was replicated just once, so we cannot estimate variation among crosses within cross types, which, however, was not a focus of our study. Fitness differences between the two main cross types were tested in a repeated measures model (GLM procedure in SAS; SAS Institute Inc., 1999) including allelic diversity, cross type, and the interaction term. To investigate separate life stages, we repeated this analysis for seed set, germination rate, the first component of a PCA reflecting F1 growth performance (number of rooted rosettes, the sum of flowers, buds, and infructescences, and wet biomass), and F1 seed production. The first eigenvector of the PCA explained 87% of the variance in the three adult fitness components (loadings: flowers=0.581, rooted rosettes=0.574, wet biomass=0.577).
We did not adjust α for multiple comparisons because we adopted a hierarchical approach, first testing whether population mean fitness varies depending on cross type and allelic diversity, and then exploring mechanisms contributing to the relationships. We log-transformed measures of fitness (natural logarithm) except for proportions, which underwent an angular transformation (Sokal and Rohlf, 1995), and offspring seed production, which remained untransformed.
Results
There was no effect of FST on mean offspring performance after between-population crosses (N=26, P>0.3 for sexual and clonal performance). Therefore, we pooled crosses with ‘near’ and ‘far’ partner populations for each target population.
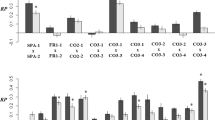
Table 1 and Figure 1 summarize and illustrate the fitness consequences of interpopulation outbreeding. Cross type had a significantly positive effect on clonal performance (average increase 16.1%; Figure 1a; untransformed data in Appendix A) and tended to increase sexual performance (average increase 34.5%). This finding is consistent with other studies suggesting that even medium-sized and large populations sometimes harbor genetic load or are slightly inbred. The positive impact of interpopulation outcrossing was especially strong for the life stage of seed set (Table 1, Figure 1b), mainly as a result of increased compatibility of genotypes of different populations in comparison with genotypes of the same population, as shown below. Offspring seed production tended to be higher in interpopulation crosses (Figure 1c).
Population means (±SE) of within- and between- population crosses for the estimate of clonal performance, and the life stages of seed set and offspring seed production (N=13; untransformed data). Test statistics are presented in Table 1.
When we excluded incompatible crosses from the analyses seed set (Table 1) and clonal performance (cross type: F1,11=4.33, P=0.0615) still tended to be higher after interpopulation outbreeding. This would suggest that the fitness benefit of interpopulation crosses was not entirely due to increased cross-compatibility, but also due in part to heterosis.
The significant interaction between cross type and allelic diversity indicates that long-term small populations experienced a higher gain in clonal performance from interpopulation outbreeding than did large populations (Table 1, Figure 2a). The clonal fitness of the seven smallest populations increased by 39.4%, mostly at the life stage of seed set (Figure 2b). After the exclusion of incompatible crosses, clonal performance still tended to be higher after interpopulation outbreeding for long-term small populations than for long-term large populations (interaction between cross type and allelic diversity: F1,11=3.56, P=0.0857). These results illustrate the efficacy of an immediate genetic rescue effect from interpopulation outbreeding, because one generation of interpopulation outbreeding can restore cross-compatibility and reduce inbreeding depression.
Changes in population mean fitness after interpopulation outbreeding for the estimate of clonal performance, and the life stages of seed set and offspring seed production, plotted against allelic diversity (N=13; untransformed data). Differences are calculated relative to within-population crosses. Test statistics are presented in Table 1.
Discussion
There is convincing empirical evidence that populations that remain small for long periods of time suffer from diverse genetic Allee effects. Small populations of our study species, Ranunculus reptans, are known to suffer from reduced cross-compatibility, inbreeding depression affecting clonal fitness, and fixed drift load for female fertility (Willi et al, 2005). Genetic Allee effects can explain the observed fitness reduction both in experimentally performed crosses among plants from small populations (Willi et al, 2005) and in clonal propagules of field-collected plants of small populations raised in a common environment (Fischer et al, 2000a). The fact that long-term small populations enjoy a higher fitness benefit from interpopulation outbreeding – as shown in this study – provides further evidence for reduced fitness in small populations. This result validates the method of comparing within- and between-population crosses to study genetic problems of populations, as suggested by Keller and Waller (2002).
Our study investigated the potential of interpopulation outbreeding to alleviate cross-compatibility, inbreeding depression, and fixed drift load. Theory predicts strong heterosis for individuals from small populations that are connected by some level of migration (Whitlock et al, 2000). In agreement with these predictions, we found that long-term small populations experience a higher gain in clonal fitness from interpopulation outbreeding than large populations. Two reasons seem to account for this result: an increase in cross-compatibility, and the reduction of inbreeding depression through heterosis.
We found a general increase in clonal performance after one generation of interpopulation outbreeding for populations independent of their long-term size. We see three possible reasons for this outcome. First, even medium-sized and large populations can have genetic load and reduced fitness as a consequence of the mutation-drift-selection balance (Lande, 1994). Second, populations often have genetic substructure, with close relatives living close together and, consequently, frequent inbreeding (Waller, 1993). In fact, populations of R. reptans are known to be genetically substructured (Fischer et al, 2000b). A third reason is that interpopulation outbreeding leads to a decreased likelihood of incompatible crosses. Our results indicate that increased cross-compatibility explains substantial, but not all, fitness benefit. Furthermore, we found a tendency for increased seed production in offspring of interpopulation crosses in the common garden, where pollen was randomly available, suggesting that female fertility was restored.
Another interesting implication of our results is that immigrant alleles would be of fitness advantage and should spread in their new populations. This is especially true for S-alleles at the incompatibility locus, which are strongly favored when rare by frequency-dependent selection (Byers and Meagher, 1992). Beyond incompatibility, theory predicts that offspring of interpopulation migrants are likely to experience heterosis due to the masking of deleterious mutations (Ingvarsson and Whitlock, 2000). This will result in immigrant alleles being present in higher frequencies than predicted from neutral expectations. Indeed, Saccheri and Brakefield (2002) found that immigrant genomes in artificially inbred butterfly populations spread due to heterosis, and that the advantage to the immigrant genes was sustained over several generations. That study, and a similar experiment with Daphnia (Ebert et al, 2002), suggest that effective migration rates, especially when populations are inbred or harbor high local drift load, may often be much higher than the number of individual migrants assumed by classical population genetics models. Furthermore, organisms may have mechanisms of gamete discrimination, preferring immigrant over inbred gametes. Richards (2000) showed that immigrant pollen in an inbred population sired a higher number of seed than expected by random mating and suggested pollen discrimination as a possible explanation. Future studies should focus on the magnitude of the spread of alleles after immigration depending on population features and on the underlying mechanisms.
Our study allows several conclusions relevant for conservation. Interpopulation outbreeding is likely to be an effective genetic restoration measure. Our study shows that the fitness benefits of interpopulation outbreeding are especially large for small populations. Moreover, gene flow into small populations would decrease population differentiation, which is more pronounced for small populations. Self-incompatible species can be expected to be rather resistant to outbreeding depression. Still, to avoid outbreeding depression, the magnitude of differential adaptation and differentiation in coadapted gene complexes between target and source populations should be considered before artificial gene flow is used as a conservation measure.
References
Brys R, Jacquemyn H, Endels P, Van Rossum F, Hermy M, Triest L et al (2004). Reduced reproductive success in small populations of the self-incompatible Primula vulgaris. J Ecol 92: 5–14.
Byers DL, Meagher TR (1992). Mate availability in small populations of plant species with homomorphic sporophytic self-incompatibiliy. Heredity 68: 353–359.
De Nettancourt D (2001). Incompatibility and Incongruity in Wild and Cultivated Plants, 2nd edn. Springer-Verlag: Berlin.
Ebert D, Haag C, Kirkpatrick M, Riek M, Hottinger JW, Pajunen VI (2002). A selective advantage to immigrant genes in a Daphnia metapopulation. Science 295: 485–488.
Fischer M, Hock M, Paschke M (2003). Low genetic variation reduces cross-compatibility and offspring fitness in populations of a narrow endemic plant with a self-incompatibility system. Conserv Genet 4: 325–336.
Fischer M, Husi R, Prati D, Peintinger M, van Kleunen M, Schmid B (2000b). RAPD variation among and within small and large populations of the rare clonal plant Ranunculus reptans (Ranunculaceae). Am J Bot 87: 1128–1137.
Fischer M, Matthies D (1998). Effects of population size on performance in the rare plant Gentianella germanica. J Ecol 86: 195–204.
Fischer M, van Kleunen M, Schmid B (2000a). Genetic Allee effects on performance, plasticity and developmental stability in a clonal plant. Ecol Lett 3: 530–539.
Frankham R (1996). Relationship of genetic variation to population size in wildlife. Conserv Biol 10: 1500–1508.
Hardy OJ, Vekemans X (2002). SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2: 618–620.
Hauser TP, Loeschcke V (1994). Inbreeding depression and mating-distance dependent offspring fitness in large and small populations of Lychnis flos-cuculi (Caryophyllaceae). J Evol Biol 7: 609–622.
Hebert PDN, Beaton MJ (1993). Methodologies for Allozyme Analysis Using Cellulose Acetate Electrophoresis: A Practical Handbook. Helena Laboratories: Beaumont, TX.
Heschel MS, Paige KN (1995). Inbreeding depression, environmental stress, and population size variation in scarlet gilia (Ipomopsis aggregata). Conserv Biol 9: 126–133.
Ingvarsson PK, Whitlock MC (2000). Heterosis increases the effective migration rate. Proc R Soc Lon Ser B 267: 1321–1326.
Jacquemyn H, Brys R, Hermy M (2002). Patch occupancy, population size and reproductive success of a forest herb (Primula elatior) in a fragmented landscape. Oecologia 130: 617–625.
Keller LF, Waller DM (2002). Inbreeding effects in wild populations. Trends Ecol Evol 17: 230–241.
Kéry M, Matthies D, Spillmann HH (2000). Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea. J Ecol 88: 17–30.
Kimura M, Maruyama T, Crow JF (1963). The mutation load in small populations. Genetics 48: 1303–1312.
Lande R (1994). Risk of population extinction from fixation of new deleterious mutations. Evolution 48: 1460–1469.
Luijten SH, Dierick A, Gerard J, Oostermeijer B, Raijmann LEL, Den Nijs HCM (2000). Population size, genetic variation, and reproductive success in a rapidly declining, self-incompatible perennial (Arnica montana) in The Netherlands. Conserv Biol 14: 1776–1787.
Lundqvist A (1990). The complex S-gene system for control of self-incompatibility in the buttercup genus Ranunculus. Hereditas 113: 29–46.
Lundqvist A (1994). The self-incompatibility system in Ranunculus repens (Ranunculaceae). Hereditas 120: 151–157.
Lynch M, Conery J, Bürger R (1995). Mutation accumulation and the extinction of small populations. Am Nat 146: 489–518.
Mavraganis K, Eckert CG (2001). Effects of population size and isolation on reproductive output in Aquilegia canadensis (Ranunculaceae). Oikos 95: 300–310.
Menges ES (1991). Seed-germination percentage increases with population size in a fragmented prairie species. Conserv Biol 5: 158–164.
Morgan JW (1999). Effects of population size on seed production and germinability in an endangered, fragmented grassland plant. Conserv Biol 13: 266–273.
Nei M (1973). Analysis of gene diversity in subdivided populations. Proc Nat Acad Sci USA 70: 3321–3323.
Oostermeijer JGB, van Eijck MW, den Nijs JCM (1994). Offspring fitness in relation to population size and genetic variation in the rare perennial plant species Gentiana pneumonanthe (Gentianaceae). Oecologia 97: 289–296.
Ouborg NJ, Van Treuren R (1994). The significance of genetic erosion in the process of extinction. IV. Inbreeding load and heterosis in relation to population size in the mint Salviapratensis. Evolution 48: 996–1008.
Paland S, Schmid B (2003). Population size and the nature of genetic load in Gentianella germanica. Evolution 57: 2242–2251.
Paschke M, Abs C, Schmid B (2002). Relationship between population size, allozyme variation, and plant performance in the narrow endemic Cochlearia bavarica. Conserv Genet 3: 131–144.
Prati D, Peintinger M (2000). Biological flora of Central Europe: Ranunculus reptans L. Flora 195: 135–145.
Richards CM (2000). Inbreeding depression and genetic rescue in a plant metapopulation. Am Nat 155: 383–394.
Saccheri IJ, Brakefield PM (2002). Rapid spread of immigrant genomes into inbred populations. Proc R Soc Lon Ser B 269: 1073–1078.
SAS Institute Inc (1999). SAS OnlineDoc®, Version 8. SAS Institute Inc: Cary, NC.
Schmidt K, Jensen K (2000). Genetic structure and AFLP variation of remnant populations in the rare plant Pedicularispalustris (Scrophulariaceae) and its relation to population size and reproductive components. Am J Bot 87: 678–689.
Severns P (2003). Inbreeding and small population size reduce seed set in a threatened and fragmented plant species, Lupinus sulphureus ssp. kincaidii (Fabaceae). Biol Conserv 110: 221–229.
Sokal RR, Rohlf FJ (1995). Biometry, 3rd edn. W.H. Freeman and Company: New York.
Van Treuren R, Bijlsma R, Ouborg NJ, van Delden W (1993). The significance of genetic erosion in the process of extinction. IV. Inbreeding depression and heterosis effects caused by selfing and outcrossing in Scabiosa columbaria. Evolution 47: 1669–1680.
Vergeer P, Rengelink R, Copal A, Ouborg NJ (2003). The interacting effects of genetic variation, habitat quality and population size on performance of Succisapratensis. Jecol 91: 18–26.
Waller DM (1993). The statics and dynamics of mating system evolution. In: Thornhill NW (ed) The Natural History of Inbreeding and Outbreeding. University of Chicago Press: Chicago. pp 97–117.
Whitlock MC, Ingvarsson PK, Hatfield T (2000). Local drift load and the heterosis of interconnected populations. Heredity 84: 452–457.
Widén B (1993). Demographic and genetic effects on reproduction as related to population size in a rare, perennial herb, Senecio integrifolius (Asteraceae). BiolJ Linn Soc 50: 179–195.
Willi Y, Van Buskirk J, Fischer M (2005). A threefold genetic Allee effect: population size affects cross-compatibility, inbreeding depression, and drift load in the self-incompatible Ranunculus reptans. Genetics 169: 2255–2265.
Wright S (1931). Evolution in Mendelian populations. Genetics 16: 97–159.
Wright S (1977). Evolution and the Genetics of Populations, Experimental Results and Evolutionary Deductions, Vol 3. University of Chicago Press: Chicago.
Acknowledgements
We thank B Rosemary Grant for discussions and advice. Thanks to Susan Hoebee, Rolf Holderegger, Burgi Liebst, and Sandy Röthlisberger for the introduction to allozyme electrophoresis; to Daniela Lang, Regula Langenauer, Evelyn Underwood, and Josh Van Buskirk for help with fieldwork. Josh Bizozzero, Esther Glaus, Simone Käppeli, Claudia Kübler, Daniela Lang, Susanne Müller, Sabine Rahm, Romain Scalone, Vanessa Summa, Rico Tuor, Gillianne Vergnerie, Andrea Weidt, Anton Willi, Claudia Willi, Edith Willi, and Marcel Zefferer gave technical assistance. Many thanks to Susan Hoebee and Josh Van Buskirk for constructive comments on the manuscript. We were supported by grant 31-67876.02 of the Swiss National Science Foundation and by the Institute of Environmental Sciences, University of Zürich.
Author information
Authors and Affiliations
Corresponding author
Appendix A
Appendix A
Means±SE of within- and pooled between-population crosses are given in Table A1.
Rights and permissions
About this article
Cite this article
Willi, Y., Fischer, M. Genetic rescue in interconnected populations of small and large size of the self-incompatible Ranunculus reptans. Heredity 95, 437–443 (2005). https://doi.org/10.1038/sj.hdy.6800732
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.hdy.6800732
Keywords
This article is cited by
-
Pollination insights for the conservation of a rare threatened plant species, Astragalus tragacantha (Fabaceae)
Biodiversity and Conservation (2019)
-
Effects of genetic distance on heterosis in a Drosophila melanogaster model system
Genetica (2018)
-
Forests and global change: what can genetics contribute to the major forest management and policy challenges of the twenty-first century?
Regional Environmental Change (2016)
-
Sterile males in a parasitoid wasp with complementary sex determination: from fitness costs to population extinction
BMC Ecology (2015)
-
Genetic and fitness consequences of interpopulation mating in Dianthus guliae Janka: conservation implications for severely depleted and isolated plant populations
Conservation Genetics (2015)