Abstract
The grape phylloxera, Daktulosphaira vitifoliae, is a viticultural pest that in the past has devastated vineyards worldwide, yet little is known about this insect's biology. The genetic structure of Australian populations of grape phylloxera and its mode of reproduction were studied following the development of four polymorphic microsatellite loci. Insects were collected from 28 vineyards, with a total of 361 insects included in the study. The majority of vineyards were infested by functionally parthenogenetic lineages of grape phylloxera that inhabit the root system and there was little support for the traditionally described holocyclic life cycle for this species. Clonal diversity was limited in all of the vineyard regions, with the exception of the Rutherglen region. A multiple founder scenario or occasional sex may contribute to diversity within the Rutherglen region. Leaf galling populations comprised classes distinct from the common genotypic classes identified on the roots, suggesting limited exchange between these groups. Implications for the management of D. vitifoliae are discussed.
Similar content being viewed by others
Introduction
Grape phylloxera, Daktulosphaira vitifoliae, is an important pest of members of the genus Vitis, specifically the European grapevine V. vinifera. Its interaction with the root system of V. vinifera is known to result in severe yield reduction and eventual vine death. The economic impact of D. vitifoliae was first realized during the 1860s when it was inadvertently introduced to the European grape-growing regions from its native North America. Grape phylloxera has subsequently spread to the majority of grape-producing countries. To reduce the impact of grape phylloxera, native American Vitis species have been used in breeding strategies to develop resistant and/or tolerant rootstocks from the late 1800s, and this is now the principal management strategy employed (Ordish, 1974). Despite the widespread use of rootstocks, the insect persists in all major grape-growing countries.
Members of the superfamily Aphidoidea (Homoptera), which include the Phylloxeridae, Aphididae, and Adelgidae, have complex life cycles incorporating various mating systems. These include sexual reproduction with subsequent apomictic parthenogenetic reproduction through the suppression of meiosis (holocyclic), obligate parthenogenetic reproduction (anholocyclic), and populations with varying degrees of commitment to sexuals, including the production of only males (androcyclic) or the production of some males and females whilst parthenogenetic production is continued (mixed strategy) (Moran, 1992; Hales et al, 1997; Dedryver et al, 1998).
Holocyclic reproduction is considered the primary life cycle mode of D. vitifoliae (Downie and Granett, 1998; Granett et al, 2001). The insect has only one host type, members of the genus Vitis. Parthenogenetic reproduction is proposed to occur on the roots and leaves of Vitis in the spring and summer months. During autumn, the population on the roots exhibits a polyphenic shift to the production of alates (winged insect). The alates disperse above ground and produce asexually the male and female sexuals that subsequently mate and lay the overwintering eggs. The female fundatrix, which hatches from the sexual egg during the following spring, moves to the leaves of the grapevine and forms a leaf gall, where parthenogenetic reproduction begins again. There is subsequent movement of parthenogenetic individuals back to the root system during the summer months. In California, grape phylloxera is considered to be present mainly on the root system and is thought to be functionally parthenogenetic due to the rarity of leaf galls and the observation that first instars can overwinter on the root system (Davidson and Nougaret, 1921).
Verification of the life cycle in commercial vineyards has been difficult because of problems in observing and sampling sexual morphs from the field (Buchanan, 1990) and the absence of leaf galling on the European grape V. vinifera. Whilst leaf galling occurs on some natural hosts (ie some American Vitis species and their hybrids), there is an incompatible interaction between the insect and leaves of V. vinifera. In Europe, grape phylloxera populations causing leaf galls are widely distributed on Vitis hybrids and sex is presumed to occur (Forneck et al, 2000). Alates have been sampled from field grapevines and sexuals observed in laboratory situations, but neither observation confirms the completion of the sexual cycle in the vineyard nor the impact of the sexual stages on the incidence of leaf galls (Adcock, 1902; Davidson and Nougaret, 1921; Forneck et al, 2001).
Studies of the genetic structure of D. vitifoliae in Europe have suggested that sex does occur in the field but that the incidence may vary between northern and southern Europe. This hypothesis was based on analysis of amplified fragment length polymorphism (AFLP) markers that revealed extensive polymorphism within and between populations, and a relatively greater level of genetic diversity in northern regions (Forneck et al, 2000). However, the dominant inheritance patterns of AFLP markers makes it difficult to quantify the contribution of meiotic processes to the observed genetic variation.
DNA markers based on microsatellite loci should be more useful for characterization of the D. vitifoliae life cycle. As codominant and highly variable DNA markers, they provide a powerful system for unraveling life history traits, particularly the occurrence of sex (reviewed in Sunnucks et al, 1997; Hales et al, 1997). They have been used in life cycle studies of members of the Aphididae family, resulting in high level resolution of reproductive mode and exploration of genetic relationships, including those of clonal lineages (Sunnucks et al, 1996, 1997; Fuller et al, 1999; Simon et al, 1999; Wilson et al, 1999).
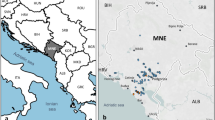
Here, microsatellite markers are used to investigate the mode of reproduction and genetic structure of grape phylloxera populations in South Eastern Australia. Since its discovery in Australia in 1877, D. vitifoliae has been identified, and contained, within five geographic regions (Figure 1) through internal quarantine measures. Australia is unique in its approach to grape phylloxera management, with a strong reliance on quarantine and the majority of vineyards not utilizing rootstocks. Based on the microsatellite DNA marker patterns obtained, three questions were posed: (i) what is the incidence of sexual and parthenogenetic life cycle strategies?; (ii) do root populations contribute to genotypes observed in leaf galling populations and vice versa?; and (iii) is there genetic diversity within and between regions and can this be related to the infestation history of grape phylloxera?
Daktulosphaira vitifoliae infested regions and sampling sites in Victoria (VIC) and New South Wales (NSW), Australia. Areas with known D. vitifoliae infestations are termed Phylloxera Infestation Zones (PIZs). The shaded regions indicate the three PIZs from which samples were collected in Victoria; Nagambie, King Valley, and North East. The Rutherglen, Glenrowan and Milawa collection sites are all located within north east Victoria PIZ. No insects were found in vineyards from the other Victorian PIZ, Mooroopna (location not shown). The samples collected from NSW are located at Orchard Hills, near Sydney. Table 1 lists the regional location of the vineyards from which D. vitifoliae samples were collected.
Materials and methods
Insect collection
Root samples were collected in January 2000 from 28 vineyards, previously reported as infested with D. vitifoliae (Buchanan, 1987). The vineyards were located within four of the five Phylloxera Infestation Zones (PIZs) located in Victoria and New South Wales (NSW), Australia (). A vineyard was defined as a single viticultural operation, owned and operated by a particular company within a region but often varying in size (Table 1).
Under the traditional life cycle of grape phylloxera, summer root samples should be produced by parthenogenesis following sexual reproduction and parthenogenesis over the preceding winter. To increase the likelihood of collecting recombinant offspring from the putative sexual phase, only single specimens were collected from the roots of an individual vine. The distance between vine samples within a vineyard varied from 10 m up to 3 km in three (NA-1, RU-1, ML-1) of the larger vineyards. The total number of samples obtained and their location within a vineyard was dependent on both the density and distribution of the infestations (ie, how readily insects were found). Both infestation density and distribution can be influenced by infestation age, soil type, host genotype, and vine health (Buchanan, 1987). Where possible, insects were collected from the root systems of different Vitis genotypes within an individual vineyard (Table 1).
During the same collection period, leaf samples were also obtained from three vineyards, each in a different region. Only one female was collected from a single leaf gall because under the traditional life cycle all females within a gall were expected to have been produced by parthenogenesis, whereas founders of the different galls should be the product of sexual reproduction. Up to 10 galls were sampled from one leaf. In Rutherglen, the vineyard contained 20 adjacent non-grafted rootstocks (Vitis genotype unknown) and galls were collected at random from the leaves of 10 vines. In Milawa, galls were collected from the leaves of four non-grafted Schwarzmann (V. rupestris × V. riparia) rootstocks. In Glenrowan, galls were collected from within a grafted vineyard block, where some of the rootstocks (Vitis genotype unknown) had produced suckers. Leaf galls were collected from five vines, each separated by a distance of over 30 m, although other vines with leaf galls were scattered throughout this block. Root samples were also collected from two vines exhibiting leaf galls in each of the three vineyards. These samples were included in the root samples. All insects were stored in 100% ethanol at −20°C.
Cloning and amplification of microsatellites
Total DNA was extracted from grape phylloxera adults using Lin and Walker's (1996) method, with modifications as described by Corrie et al (1997), yielding approximately 5 μg of DNA from 500 insects. The insects used in the DNA extraction procedure were collected from sterile in vitro cultures to ensure minimal contamination of DNA from other organisms (Kellow et al, 1999). The construction of each partial genomic library utilized 2 μg of DNA digested with one of the following enzyme combinations: Library 1, MboI; Libraries 2, 3 and 4: Sau3AI/RsaI. The fragments were size-selected (200–700 bp) by agarose gel electrophoresis and ligated into either pBlueScript SK II (+) (Stratagene) or pUC19 vector (Genesearch) digested with BamHI. After transformation into E. coli strain JM109 (Promega), colonies were transferred to HyBond™ N+ nylon membranes (Amershem Pharmacia Biotech) according to standard methods (Ausubel et al, 1996) and probed by hybridization with [α33P]-dATP (10 mCi/ml; Geneworks) end-labelled oligonucleotides. The synthetic oligonucleotides (CA)10, (AG)10, (CCT)5, (CAC)5, (ATT)5, (ATCT)4 or (TGTA)4 were used. A total of approximately 30000 recombinants were screened from the four libraries. Plasmid inserts from the putative positives were sequenced using both standard manual and automated methods. Ten clones were identified as containing nucleotide repeat regions, however only five of these contained repeat regions consisting of more than one nucleotide base or greater than six repeat units. Primer pairs based on unique non-repetitive flanking DNA sequences could only be designed for four clones, however all of these revealed polymorphisms when used in the polymerase chain reaction (PCR) on grape phylloxera genomic DNA (Table 2). The sizes of the alleles were determined using a standard sequencing reaction of the pUC19 vector (Genesearch) as the molecular weight standard ladder.
In preparation for PCR, 5% Chelex® 100 (BioRad) was used for DNA extractions from single adult females (Walsh et al, 1991). In brief, insects were placed into a sterile 0.5 ml tube, the excess ethanol evaporated, and then ground with a pestle in liquid nitrogen, after which 60 μl 5% Chelex® 100 and 1 μl proteinase K (14 mg/ml; Boehringer Mannheim) was added, followed by incubation at 56°C for 10–12 h, then 92°C for 5 min. DNA extractions were stored at −20°C.
PCR was performed in a 10 μl volume containing 10 pmol of each primer pair, with the forward primer end-labeled with [α33P]-dATP (10 mCi/ml; Geneworks), 0.7 mM of the four dNTPs (Biotech), 1 μl 10 × PCR buffer containing 1.5 mM MgCl2 (Promega), 0.8 units Taq DNA polymerase (Promega) and 2 μl DNA. PCR consisted of an initial denaturation phase at 93°C for 2 min, followed by 30 cycles of 93°C for 30 s, annealing at the temperature indicated in Table 2 for 30 s, 72°C for 30 s, with a final extension phase at 72°C for 3 min. Electrophoresis of the PCR products was performed using standard techniques on 5% polyacrylamide gels using 1 × TBE buffer. After electrophoresis the gel was dried and exposed to X-ray film (Kodak).
Genetic analysis of root and leaf samples
Following Sunnucks et al (1997), alleles and genotypes are used with reference to single loci, whilst genotypic class refers to the alleles at all four loci. BIOSYS-1 (Swofford and Selander, 1981) was used to calculate the mean number of alleles per locus, observed direct count heterozygosity (HDC) and the expected heterozygosity (HE) based on the unbiased method (Nei, 1978). GENEPOP (Version 3.1) was used to calculate the exact test values for deviations from Hardy-Weinberg (Raymond and Rousset, 1995). The distribution of genotypic class across samples could not be compared using standard contingency tests due the small sample sizes. Instead, Monte Carlo tests were used to compare samples with the procedure from SPSS Version 10.0.
Results
The isolation of microsatellite loci proved difficult in grape phylloxera. It is unknown if the low numbers of microsatellites observed in this study is a characteristic common to other members of the Phylloxeridae. Differences in microsatellite density have been reported in other inveterbrate groups (Estoup and Angers, 1998). The four loci isolated were characterized for 361 insects from the 28 vineyards sampled. Based on these a total of 45 genotypic classes of D. vitifoliae were identified (Table 3).
For the root samples, all four loci were polymorphic for all samples, except for locus DVIT4 in the Nagambie and Orchard Hills regions (Table 4). Regions varied in the level of genetic heterogeneity, with the mean number of alleles observed per locus ranging from 1.8 in Nagambie and Orchard Hills to 4.3 in the Rutherglen region. The number of genotypic classes also varied between populations, from one in Nagambie, Orchard Hills and the King Valley to twenty-six in Rutherglen (Table 5).
Since only a few genotypic classes were found in the regions and because for all regions and loci there were significant departures from Hardy-Weinberg equilibrium (Table 4), the predominant mode of reproduction in grape phylloxera from roots appears to be parthenogenesis. In fact, the presence of a single highly heterozygous genotype in several regions indicates that parthenogenesis is often likely to be the sole method of reproduction.
The majority of the root samples were comprised of two genotypic classes, G1 and G4 (55.8% and 12.6% of total root samples, respectively). G1 was the most widespread, occurring in four regions and in the majority of vineyards within those regions (17/22). G4 was present in all vineyards sampled from Milawa and the King Valley and one vineyard in Glenrowan. These regions are adjacent in north east Victoria (Figure 1). Despite being widespread the G1 and G4 genotypic classes only co-occurred in one vineyard (GR-1). Of the other 29 genotypic classes identified from root samples, 25 were found in the Rutherglen region. Monte Carlo tests demonstrated significant differences among the regions (P < 0.001). In the Rutherglen region there was also significant heterogeneity among vineyards (P < 0.001).
Leaf gall samples in each of the three regions included many genotypic classes not sampled from the roots (Table 6) and Monte Carlo tests indicated significant (P < 0.001) differences between samples in all three regions. In fact there was only limited overlap between genotypic classes sampled in roots and leaves. In Glenrowan, most root samples were G1 (Table 5) but this class was absent in leaf samples. Similarly, in Milawa most root samples were G4 (Table 5) including those from the area where leaf samples were obtained, but this class did not occur in the leaf samples. Leaf gall samples also differed significantly among the regions using a Monte Carlo test (P < 0.001).
Even though only a single female was sampled from a leaf gall and the founders of galls were expected to be the product of sexual reproduction, there were highly significant departures from Hardy-Weinberg equilibrium in these samples (Table 4). This suggests that gall samples do not arise directly from sexual reproduction.
Discussion
Phylloxera reproduction
Two genotypic classes, G1 and G4, predominated in root samples across different geographic regions and these genotypic classes were heterozygous at three of the four loci and all loci respectively. This indicates that the majority of the Australian populations of grape phylloxera are comprised of functionally parthenogenetic lineages. The heterozygous nature of these classes at several loci is consistent with previous observations of other primarily parthenogenetic aphid populations (Fuller et al, 1999; Simon et al, 1999). Reproduction by parthenogenesis in these lineages is supported by the absence of expected recombinant genotypes, such as individuals that are homozygous for the DVIT1128 allele and significant deviation from Hardy-Weinberg equilibrium for all loci (Table 4).
G1 and G4 appear very similar and could only be differentiated by one allele at the DVIT4 locus (Table 3). The difference in size between these DVIT4 alleles was 9 bp, consistent with it being generated by the slip-strand mispairing (SSM) mechanism of microsatellite evolution, the DVIT4 locus containing a perfect tri-repeat unit (Eisen, 1999). This suggests G1 and G4 are derived from a common lineage. Only one other genotypic class, G16, may share a relatively recent origin with G1 and G4 (Table 3). No other derivatives of G1 were observed, despite being expected from the SSM model.
The traditional model of the life cycle of D. vitifoliae describes the leaf galling populations as originating each season from fundatrixes, which result from sexual recombination between individuals derived from the root populations. However, the data presented here indicate that this is not the primary life cycle strategy employed by populations of D. vitifoliae in Australian vineyards. Leaf galls were only observed in three of the 28 vineyards where grape phylloxera were sampled, and in those vineyards where both root and leaf populations co-exist there is little evidence that the leaf gall genotypes result from sexual recombination between the root genotypes sampled. For instance, consider the Glenrowan (GR-1) data where four genotypic classes were identified in root samples and five in leaf gall samples (Tables 5 and 6). Two of the root classes, G2 and G3, are identical to the most common leaf genotypic classes. Migration of clones between roots and leaves is a more probable reason for this observation than the generation of identical genotypes in leaf and root populations via independent sexual recombination events. The leaf gall genotypic class G38 could be the outcome of sex between individuals of the genotypic classes G2 and G3 (Table 3). However, additional genotypic classes comprised of DVIT1136 homozygotes could also be expected to result from sex between G2 and G3 genotypic classes, but were not identified in any leaf gall sample.
The absence of G1 and G4 in the leaves in this region and the other regions suggests that these classes are restricted to the roots. Given that these classes are widespread and predominate in recently infested vineyards, they may well have biological attributes that preadapt them to colonizing new areas.
Population structure
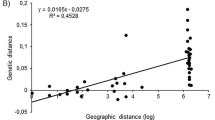
Many genotypic classes were observed in the Rutherglen region root samples in contrast to other regions. This raises the issue of whether this diversity is a consequence of rare sexual recombination and/or multiple introductions possibly coupled with mutation. Recent sexual reproduction does not appear to be the major factor as identical genotypic classes occur in more than one vineyard. If the genotypes were the result of recombination followed by expansion of lineages by parthenogenesis, the majority of samples of any one genotypic class should be found within the same location in the presence of related recombinant genotypes, particularly as grape phylloxera has limited dispersal under Australian conditions (King and Buchanan, 1986). However, of the 15 genotypic classes sampled multiple times, nine were found in more than one vineyard (Table 5). The likelihood of these identical genotypic classes arising in separate locations via independent sexual recombinant events is low, unless there was extremely rare sex with sexual offspring unable to compete successfully against parthenogenic lineages (Lynch, 1984; Wilson et al, 1999; Peck and Waxman, 2000). More intensive intra-vineyard studies within the Rutherglen region should help to ascertain if rare sex and recombinant genotypic classes do indeed occur.
Another explanation for the relatively high level of genetic diversity in the Rutherglen region involves multiple introductions of clones of polyphyletic origins. Historical records of grapevine movement suggest the Rutherglen region was a site of extensive introductions of grapevine material from Europe during the late-18th and early-19th Centuries (reviewed in Corrie et al, 1997). The insect was widespread in Europe during this time and several genotypes could have been transported to Australia on grapevine material. Recent AFLP studies have demonstrated a high degree of genetic variability within European D. vitifoliae populations (Forneck et al, 2000). With the detection of downy mildew (Plasmopara viticola) in Australia in 1917, strict quarantine restrictions were implemented on imported grapevine material (de Castella and Brittlebank, 1917). Consequently, new grape phylloxera introductions into Australia would be extremely unlikely.
However, this scenario is inconsistent with the low diversity in other regions in Australia, which have long viticultural histories. Continuous viticulture has occurred in Nagambie since D. vitifoliae was first observed during the 1890s, and in Glenrowan and Milawa since the insect was observed during the 1920s, providing an opportunity for founder population continuation. Movement of vine material has occurred between these regions and the Rutherglen region over the last 80 years. Until 1986, all these regions were included in one large quarantine district enabling the free movement of grapevine material and consequently any grape phylloxera present on this material (Buchanan, 1987). Other factors therefore appear to be influencing the frequency and geographic distribution of clones.
Host specialisation has been implicated in the maintenance of high intra-population variation of some aphid species (De Barro et al, 1995; Hales et al, 1997). Previous studies have implied D. vitifoliae populations do differ in their ability to inhabit various Vitis genotypes (Granett et al, 1985; King and Rilling, 1985), although DNA typing data collected so far suggest a limited association of host plant with particular genetic strains in both native and cultivated vineyard situations (Downie, 2000; Forneck et al, 2000).
A difference in clonal heterogeneity over an environmental range has been described in other organisms including unisexual fish (Vrijenhoek and Pfeiler, 1997) and snails (Fox et al, 1996). In these organisms, the frozen niche variation model has been invoked to help explain the observation that clonal lineages could coexist in space (Vrijenhoek, 1979). Essentially the model suggests that clonal diversity is maintained by selection due to environmental variation with occasional sex, resulting in ‘specialised’ clones adapted to particular niches. Decreased clonal diversity would then be expected in environments with reduced variation. However, the results of our study are inconsistent with this model at least in terms of heterogeneity associated with vine host. For example, in the vineyard RU-3 only one vine host was present (AxR#1) but various genotypic classes of grape phylloxera were found.
The occurrence of G1 over a broad geographic range suggests that this clone may be a ‘general purpose genotype’ (Lynch, 1984). Clones with low fitness variance for a large range of biotic and environmental factors may result from selection on populations with a long history of clonal reproduction. While these generalised genotypes may be out competed in the short term in niche environments by more specialised clonal or sexual lineages, over time they will be present over the broadest geographic range. Nevertheless, grape phylloxera is an introduced species to Australia and the genotypic pattern could be the result of founder effects rather than the biological attributes of clonal lineages. A more comprehensive study on the genetic relationship between grape phylloxera clones and their relative fitness will help to resolve the potential contribution of the above models to clonal persistence, composition and distribution.
Applied significance
The predominance of parthenogenetic reproduction on the root system has implications for the use of rootstocks. Increasingly the durability of Vitis host resistance in commercial viticulture has been questioned, with the suggestion that more virulent strains of grape phylloxera may arise through the processes of evolution and selection (Granett et al, 1985; Bleser and Rühl, 1997). The absence of sexual offspring in the root gall populations may reduce the risk of resistance adaptation in the field, although somatic mutation could still have an impact on resistance evolution.
With regards to quarantine, the lack of recombinant genotypes on the roots indicates that alates, and consequently sexuals, are not likely to be a source of spread of this insect. Likewise, leaf galls do not appear to be the major quarantine risk, since there appears to be little interaction between leaf and root populations (ie, insects from leaf galls rarely establish on roots). Indeed the data indicate that G1 and G4 genotypes should be the focus of quarantine studies, being the most widespread of the genotypic classes. The parthenogenetic root gall insects appear to be of higher quarantine risk than the alates, sexual forms, or those from leaf galls.
References
Adcock, GH (1902). The History of Phylloxera in Victoria. Department of Agriculture Victoria: Melbourne, pp 79–83.
Ausubel, F, Brent, R, Kingston, R, Moore, D, Seidman, JG, Smith, JA et al (1996). Current Protocols in Molecular Biology. John Wiley and Sons: Brooklyn, New York.
Bleser, E, Rühl, E (1997). Schädling gibt noch viele Rätsel auf. Das Deutsche Weinmagazin, 10–17 Mai, pp 22–26.
Buchanan, GA (1987). The distribution of grape phylloxera,Daktulosphaira vitifoliae (Fitch), in central and north-easternVictoria. Aust J Exper Agric, 27: 591–593.
Buchanan, GA (1990). The distribution, biology and control of grape phylloxera, Daktulosphaira vitifolii, (Fitch), in Victoria. PhD thesis, La Trobe University, Melbourne.
Corrie, AM, Buchanan, GA, van Heeswijck, R (1997). DNA typing of populations of phylloxera (Daktulosphaira vitifoliae (Fitch)) from Australian vineyards. Aust J Grape Wine Res, 3: 50–56.
Davidson, WM, Nougaret, RL (1921). The grape phylloxera in California. United States Department of Agriculture (Washington), pp 1–28.
De Barro, PJ, Sherratt, TN, David, O, Maclean, N (1995). An investigation of the differential performance of clones of the aphid Sitobion avenae on two host species. Oecologia, 104: 379–385.
de Castella, F, Brittlebank, C (1917). Note on Downy Mildew. J Agric (Victoria), 15: 685–700.
Dedryver, CA, Le Gallic, JF, Gauthier, JP (1998). Life cycle of the cereal aphid Sitobion avenae F.: polymorphism and comparison of life history traits associated with sexuality. Ecol Entomol, 23: 123–132.
Downie, D (2000). Patterns of genetic variation in native grape phylloxera on two sympatric host species. Mol Ecol, 9: 505–514.
Downie, D, Granett, J (1998). A life cycle variation in Grape Phylloxera Daktulosphaira vitifoliae (Fitch). Southwest Entomol, 23: 11–16.
Eisen, JA (1999). Mechanistic basis for microsatellite instability. In: Goldstein DB, Schlötterer C (eds) Microsatellites: Evolution and Application, Oxford University Press: Oxford, pp 35–48.
Estoup, A, Angers, B (1998). Microsatellites and minisatellites for molecular ecology: theoretical and empirical considerations. In: Carvalho GR (ed) Advances in Molecular Ecology, IOS Press: Amsterdam, pp 55–86.
Fletcher, MJ (1984). The occurance of phylloxera in New South Wales. In: Buchanan GA, Amos TA (eds) The Biology, Quarantine and Control of Grape Phylloxera in Australia and NewZealand, Victorian Deparrment of Agriculture: Rutherglen,Victoria, Australia, p 78.
Forneck, A, Walker, MA, Blaich, R (2000). Genetic structure of an introduced pest, grape phylloxera (Daktulosphaira vitifoliae Fitch), in Europe. Genome, 43: 669–678.
Forneck, A, Walker, MA, Blaich, R (2001). An in vitro assessment of phylloxera (Daktulosphaira vitifoliae Fitch) (Homoptera Phylloxeridae) life cycle. J Appl Entomol, 125: 443–447.
Fox, JA, Dybdahl, MF, Jokela, J, Lively, CM (1996). Genetic structure of coexisting sexual and clonal subpopulations in a freshwater snail (Potamopyrgus antipodarum). Evolution, 50: 1541–1548.
Fuller, S, Chavigny, P, Lapchin, L, Vanlerberghe-Masutti, F (1999). Variation in clonal diversity in glasshouse infestations of the aphid, Aphis gossypii Glover in Southern France. Mol Ecol, 8: 1867–1877.
Granett, J, Timper, P, Lider, LA (1985). Grape Phylloxera (Daktulosphaira vitifoliae) (Homoptera: Phylloxeridae) Biotypes in California. J Econ Entomol, 78: 1463–1467.
Granett, J, Walker, MA, Kocsis, L, Omer, AD (2001). Biology and management of grape phylloxera. Ann Rev Entomol, 46: 387–412.
Hales, DH, Tomiuk, J, Wöhrmann, K, Sunnucks, P (1997). Evolutionary and genetic aspects of aphid biology: a review. Eur J Entomol, 94: 1–55.
Kellow, AV, Corrie, AM, van Heeswijck, R (1999). Surface sterilisation of phylloxera eggs for investigating grapevine-phylloxera interactions in tissue culture. Aust J Grape Wine Res, 5: 27–28.
King, PD, Buchanan, GA (1986). The dispersal of phylloxera crawlers and spread of phylloxera infestations in New Zealand and Australian vineyards. Am J Enol Viticult, 37: 26–33.
King, PD, Rilling, G (1985). Variations in the galling reaction of grapevines: Evidence of different phylloxera biotypes and clonal reaction to phylloxera. Vitis, 24: 32–42.
Lin, H, Walker, MA (1996). Extraction of DNA from a single egg of grape phylloxera (Daktulosphaira vitifoliae Fitch) for use in RAPD testing. Vitis, 35: 87–89.
Lynch, M (1984). Destabilizing hybridization, general-purpose genotypes and geographic parthenogenesis. Quart Rev Biol, 59: 257–290.
Moran, N (1992). The evolution of aphid life cycles. Ann Rev Entomol, 37: 321–348.
Nei, M (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89: 583–590.
Ordish, G (1974). The Great Wine Blight. Sidgewick and Jackson: London.
Peck, JR, Waxman, D (2000). What's wrong with a little sex? J Evol Biol, 13: 63–69.
Raymond, M, Rousset, F (1995). GENEPOP (version 1.2): a population genetics software for exact tests and ecumenicism. J Hered, 86: 248–249.
Simon, J, Baumann, S, Sunnucks, P, Hebert, P, Pierre, J, Le Gallic, JF et al (1999). Reproductive mode and population genetic structure of the cereal aphid Sitobion avenae studied using phenotypic and microsatellite markers. Mol Ecol, 8: 551–545.
Sunnucks, P, de Barro, PJ, Lushai, G, Maclean, N, Hales, D (1997). Genetic structure of an aphid studied using microsatellites: cyclic parthenogenesis, differentiated lineages and host specialization. Mol Ecol, 6: 1059–1073.
Sunnucks, P, England, PR, Taylor, AC, Hales, DF (1996). Microsatellite and chromosome evolution of parthenogenetic Sitobion Aphids in Australia. Genetics, 144: 747–756.
Swofford, DL, Selander, RB (1981). BIOSYS-1: A FORTRAN program for the comprehensive analysis of electrophoretic data in population genetics and systematics. J Hered, 72: 281–283.
Vrijenhoek, RC (1979). Factors affecting clonal diversity and coexistance. Am Zool, 19: 787–797.
Vrijenhoek, RC, Pfeiler, E (1997). Differential survival of sexual and asexual Poeciliopsis during environmental stress. Evolution, 51: 1593–1600.
Walsh, P, Metzger, D, Higushi, R (1991). Chelex® 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques, 10: 506–513.
Wilson, A, Sunnucks, P, Hales, D (1999). Microevolution, low clonal diversity and genetic affinities of parthenogenetic Sitobion aphids in New Zealand. Mol Ecol, 8: 1655–1666.
Acknowledgements
We thank Dr Steven Frankenburg, Dr Janet Hatt and Stephen Winnall for excellent assistance in the field and laboratory, and numerous colleagues for assistance in collection of samples, in particular Rebecca Dunstone, Shane Hackett and Megan Hill. The cooperation of the vineyard owners involved was much appreciated. Thanks to Dr Michael Goodismann, Tracy Reynolds, and two anonymous reviewers for comments on the manuscript. Financial support was provided by the Phylloxera and Grape Industry Board of South Australia, the Department of Natural Resources and Environment, and the Australian Research Council via their SPIRT and Special Research Centre Programs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corrie, A., Crozier, R., Van Heeswijck, R. et al. Clonal reproduction and population genetic structure of grape phylloxera, Daktulosphaira vitifoliae, in Australia. Heredity 88, 203–211 (2002). https://doi.org/10.1038/sj.hdy.6800028
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.hdy.6800028
Keywords
This article is cited by
-
A diagnostic LAMP assay for the destructive grapevine insect pest, phylloxera (Daktulosphaira vitifoliae)
Scientific Reports (2020)
-
Major Outbreaks in the Nineteenth Century Shaped Grape Phylloxera Contemporary Genetic Structure in Europe
Scientific Reports (2019)
-
First European leaf-feeding grape phylloxera (Daktulosphaira vitifoliae Fitch) survey in Swiss and German commercial vineyards
European Journal of Plant Pathology (2019)
-
Genetic identification of SNP markers linked to a new grape phylloxera resistant locus in Vitis cinerea for marker-assisted selection
BMC Plant Biology (2018)
-
No evidence of superclones in leaf-feeding forms of austrian grape phylloxera (Daktulosphaira vitifoliae)
European Journal of Plant Pathology (2015)