Abstract
Purpose
To compare the measurements of intraocular pressure (IOP) with dynamic contour tonometry (DCT) and Goldmann applanation tonometry (GAT) in healthy and glaucomatous eyes and to evaluate the influence of corneal thickness (CCT), corneal curvature (CC), axial length (AL), and age on these tonometric alternatives.
Methods
Three hundred eyes of 100 healthy subjects, 100 patients with primary open angle glaucoma, and 100 patients with primary angle-closure glaucoma underwent IOP evaluation with DCT and GAT, and measurements of CCT, CC, and AL. Bland–Altman plots were used to evaluate the agreement between tonometers. Regression analysis was used to evaluate the influence of ocular structural factors on IOP measurements obtained with both tonometers.
Results
Bland–Altman plots indicated that the 95% limits of agreement between tonometers were −1.4 to 6.6 mmHg. DCT values measured 2.6±1.9 mmHg higher than GAT readings (P<0.001). The mean IOP difference between DCT and GAT (ΔIOP) was higher in healthy than in glaucomatous eyes and the magnitude of difference increased with increasing CCT. A significant reduction of ΔIOP with an increase in both CCT (P<0.001) and IOP values (P<0.001) was found. Regression analysis showed no effect of CC, AL, and age on both DCT and GAT readings. In contrast to GAT (P<0.001), DCT measurements were not influenced by CCT (P=0.43).
Conclusions
IOP readings obtained by DCT were higher and less affected by CCT than those by GAT. The ΔIOP was higher in healthy than in glaucomatous eyes and decreased in relation to increased CCT and IOP values.
Similar content being viewed by others
Introduction
Over the past four decades, the Goldmann applanation tonometry (GAT) has been the gold standard, against which other tonometers have been compared for routine measurements of intraocular pressure (IOP). However, the accuracy of GAT depends on many ocular structural factors, including central corneal thickness (CCT), corneal curvature (CC), and axial length (AL).1 Measurements of IOP made with applanation tonometry are influenced directly by the CCT. We assume and apply a single value for CCT (520 μm) in applanation tonometry estimates, although there is compelling evidence that CCT varies between individuals, with ethnicity, gender, age, and the presence of pseudoexfoliation of the lens capsule,2 and has a diurnal variation.3 Goldmann himself recognized that the tonometer's accuracy was questionable in corneas that were not of average thickness. In recent years, different studies demonstrated that changes in corneal structure and thickness, which are both altered by corneal refractive surgery, might be associated with clinically significant underestimates of IOP when measured with GAT.4, 5, 6, 7, 8, 9, 10, 11 Moreover, many cases of normal-tension glaucoma and ocular hypertension have been erroneously diagnosed by underestimation and overestimation of IOP by GAT in patients whose corneas are thinner or thicker than average, respectively.12, 13, 14, 15 Dynamic contour tonometry (DCT), a novel digital nonapplanation contact tonometer, has been proposed as an alternative method for obtaining IOP readings significantly closer to true manometric levels.16 DCT was designed to be largely independent of structural properties of the cornea and to eliminate most of the systematic errors arising from physiological variables of the eye, which render GAT less accurate.17 Some recent studies compared the performance of the new device against other tonometers18, 19, 20, 21, 22 considering either the influence of pachymetry on IOP readings23, 24, 25, 26 or the impact of refractive surgery as a source of errors in applanation tonometry.27, 28, 29, 30 Only two studies evaluated ocular structural factors other than pachymetry influencing IOP measurements in healthy subjects31 or African American subjects.32 In this study, we compared the IOP readings obtained by DCT with those of GAT and analysed the influence of ocular structural factors (CCT, CC, AL) and age on IOP readings in a large group of Caucasian healthy subjects, patients with primary open angle glaucoma (POAG), and patients with primary angle-closure glaucoma (PACG). Moreover, the effect of CCT, CC, AL, IOP, and age on ocular pulse amplitude (OPA) measurements was assessed.
Materials and methods
One hundred healthy subjects from the hospital staff, 100 patients with POAG, and 100 patients with PACG, all attending their routine clinical appointment at the Glaucoma Service at the Eye Clinic of the University of Verona, contributed 300 eyes that were evaluated in a cross-sectional study. Each participant underwent all the measurements in one randomly selected eye. The Institutional Review Board at the University of Verona approved the study protocol and all study procedures conformed to the Declaration of Helsinki for research involving human subjects. After informed consent was obtained, participants underwent a complete ophthalmic examination including a review of medical history, best-corrected visual acuity assessment, slit-lamp examination, gonioscopy, and fundus biomicroscopy with a +78 D lens. The inclusion criteria included best-corrected visual acuity of 0.7 or better and the absence of ocular pathologies other than glaucoma. The exclusion criteria included corneal astigmatism higher than 2 D, corneal diseases, microphthalmos, cataract greater than mild lens opacity (according to the Lens Opacity Classification System III),33 history of intraocular or refractive surgery, ocular inflammation, and contact lens wear. The patients were classified as glaucomatous if they had IOP higher than 21 mmHg before medications, optic disc with typical glaucomatous findings (cup/disc asymmetry between two eyes ⩾0.2, neuroretinal rim thinning, notching, disk haemorrhage or nerve fibre layer defects), repeatable and reliable abnormal visual field results using the automated perimetry performed with the 24-2 Swedish Interactive Threshold Algorithm (HFA; Carl Zeiss Meditec, Dublin, CA, USA). Reliable visual fields were required to have fixation losses, false-positive results, and false-negative results below 20%. An abnormal visual field was defined as a pattern SD with P<5%, glaucoma hemifield test outside normal limits, or both. The 100 healthy participants were enrolled after a complete ophthalmic examination and a thorough history taking, which confirmed no history of previous ocular surgery and the absence of ocular pathologies. The 100 glaucomatous patients affected by POAG were selected from those who were treated only with topical IOP-lowering medications (β-blockers, carbonic anhydrase inhibitors, prostaglandin analogues, α2-agonists) and who did not show peripheral anterior synechiae (PAS) after gonioscopy. The 100 patients with PACG were selected from those who were receiving topical miotic therapy (pilocarpine 2%), alone or in association with other antiglaucoma topical medication, who showed PAS, and who had never undergone previous ocular surgery except for peripheral laser iridotomy. Each participant underwent all the subsequent measurements, which were obtained by the same experienced examiner (PC) in one randomly selected eye, in the following order: biometry, tonometry, and pachymetry. AL and CC were both obtained by optical biometry system (IOL Master; Carl Zeiss AG, Feldbach, Switzerland). IOP was measured first as any manipulation to the eye before tonometry (contact ultrasonic pachymetry) could alter the pressure readings. All measurements with GAT (Haag-Streit International, Köniz, Switzerland) and DCT (Pascal dynamic contour tonometer; Swiss Microtechnology AG, Port, Switzerland) tonometer were taken by the same experienced examiner (PC), who was masked to the readings obtained. A different observer (RM) was used to read and record the IOP readings from both to minimize any potential form of bias. IOP was measured with GAT and DCT tonometer in random order, with a 5-min time interval between readings, to avoid the possibility of IOP fluctuation due to diurnal variations. GAT was assembled on a slit lamp (Haag-Streit International) and calibrated according to the manufacturer's guidelines before each participant underwent tonometry. Three consecutive IOP measurements were obtained (paper strips impregnated with fluorescein were used to stain the precorneal tear film) and averaged. Before each reading, the measuring drum was reset to approximately 6 mmHg. If IOP fluctuated during the cardiac pulse cycle, the measurement was recorded in the maximum of the pulsation amplitude (systolic phase). DCT, which automatically provides digital readout values (IOP; OPA, which is the difference between the minimum and maximum values of the pulsatile IOP wave contour during the cardiac cycle; Q-value, the qualitative score of measurement) on a liquid crystal display, was assembled on the same slit lamp and three consecutive reliable IOP readings were obtained and averaged. The corresponding OPA and Q-values were also recorded and the mean of the three readings were calculated for each eye. Particular attention was paid to standardizing the technique of DCT. The DCT tip contacts the cornea in a manner similar to that of GAT, except that correct positioning on the cornea is confirmed by an audible regular flickering signal that changes in pitch with changes in pressure. In our study, the observer (RM) recorded three consecutive readings for each eye only after she was sure that the examiner (PC) had obtained a correct IOP measurement, according to the following criteria: (1) the interrupting coupling procedure, which allows a correct calculation of the values by the device, was never performed before the audible regular flickering signal emitted at least seven consecutive pulsations; (2) the accuracy of each measurement was judged using the qualitative score provided by the device and all IOP readings were recorded only if the Q-value ranged from 1 to 3 (Q=1, optimum; Q=2 or 3, acceptable; Q=4, questionable; Q=5 or 6, repetition recommended).
Central corneal thickness was measured with an ultrasonic pachymeter (Pachette DGH 500; DGH Technology Inc., Exton, PA, USA) and the probe was placed on the centre of the cornea, over an undilated pupil, after the administration of topical anaesthesia with oxybuprocaine 0.4%. CCT was measured three times consecutively and the mean of the three readings within a range of ±5 μm was calculated for each eye.
Statistical analysis was performed with SPSS statistical software (version 13.0; SPSS Science Inc., Chicago, IL, USA) and data were presented as the mean±SD (P<0.05 was considered significant). The comparison between all the IOP measurements by DCT and GAT was performed using the nonparametric Wilcoxon signed ranked test. Furthermore, Bland–Altman plots were used to evaluate the agreement between IOP measurements obtained by DCT and GAT. The difference between measurements obtained by DCT and GAT were plotted against their mean. The method of Bland–Altman states that 95% range of agreement should be determined and then judged for its range of clinical significance.34 To examine the possible effects of pachymetry on the tonometer measurements, patients were stratified according to CCT in intervals (CCT ⩽530 μm (n=94); CCT between 531 and 560 μm (n=109); CCT >560 μm (n=97)) for statistical analysis purposes. To divide the whole sample into three groups with the same number of eyes, the thirty-third (533 μm) and the sixty-sixth (558.7 μm) percentiles were estimated and used as the cutoff values. Then we assessed the IOP measurement of DCT by comparing it to GAT with respect to multiple CCT and various groups of eyes, using the Student's paired t-test. Linear regression analysis was used to evaluate the influence of ocular structural factors (CCT, CC, AL) and age on IOP measurements obtained with both tonometers. Furthermore, the Pearson's bivariate analysis was assessed by correlating the ΔIOP (which was obtained by calculating the difference (DCT−GAT) of the IOP measurements for each patient and then the average of the 300 differences along with the SD) with the range of both CCT and IOP values in the study population. Linear regression analysis was also used to analyse the effect of CCT, CC, AL, IOP, and age on OPA measurements.
Results
The study included 300 eyes of 300 Caucasian participants from three different groups. All measurements were obtained on 300 randomly selected eyes. The main characteristics of each group are listed in Table 1. The accuracy of the 900 IOP measurements obtained with DCT in our study was high. The mean Q-value in the whole study sample was 1.9±0.7. The examiner achieved a mean optimum value (Q=1) in 63.4% of measurements and an acceptable value (Q=2 or 3) in 36.6% of them.
The concordance between the two different techniques was high. Mean IOP values obtained by DCT and GAT were strongly correlated in all eyes (r=0.93, P<0.001). However, IOP readings were significantly higher with DCT than with GAT. The assessment of the whole study population revealed a ΔIOP of +2.6±1.9 mmHg (range=−4.9 to 8.8 mmHg) (P<0.001). IOP values provided by DCT were higher than those by GAT measurements in 273 eyes (91%), lower in 26 eyes (8.7%), and equal in 1 eye (0.3%). Considering each group separately, the results showed a significantly higher ΔIOP in normal subjects than in patients with PACG (P<0.001), but not with respect to patients with POAG (Table 1).
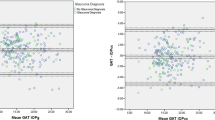
The Bland–Altman plot is shown in Figure 1. These plots allow us to investigate the existence of any systematic difference between the measurements. The average of the IOP measurements by the two methods is plotted on the x axis and the difference (DCT−GAT) on the y axis. The mean difference is the estimated bias and the SD of the differences measures the random fluctuations around this mean. The plot shows that the mean difference (DCT−GAT) is positive and that very few points fall outside the boundary limits (95% range of agreement).
Bland–Altman plot of the agreement between dynamic contour tonometry (DCT) intraocular pressure (IOP) measurements (mmHg) and Goldmann applanation tonometry (GAT) IOP measurements. The difference between the measurements is plotted against the average of the measurements. Solid line: average of the within person differences of DCT−GAT. Broken lines: 95% limits of agreement.
The analysis of the IOP measurements obtained by DCT and GAT in the subgroup of eyes whose CCT was within 531 and 560 μm revealed a significant difference only between healthy subjects and patients with PACG (P=0.009). The evaluation of the two tonometers in the subgroup of eyes with thicker corneas (CCT >560 μm) showed a significant difference either between normal subjects and patients with POAG (P=0.001) or between normal subjects and patients with PACG (P=0.03). On the contrary, the comparison of DCT with GAT in the subgroup of eyes with thinner corneas (CCT ⩽530 μm) failed to show any significant difference between healthy and glaucomatous eyes, either with open-angle or angle-closure phenotypes (Table 2).
Furthermore, the Pearson's bivariate analysis was assessed by correlating the ΔIOP with the range of both CCT and IOP values in the study population. The results revealed a significant reduction of ΔIOP with increasing CCT (P<0.001) (Figure 2). Considering the values recorded in our limited study population, we found an inverse association between DCT and GAT (IOP readings lower with DCT than with GAT) for CCT values above 620 μm either in healthy or in glaucomatous subjects (Figure 3). We also found a significant decrease of ΔIOP with an increase in IOP value (P<0.001) (Figure 4). The analysis of the data showed an analogous inversion of the performances of both tonometers for IOP values above 24 mmHg. According to this analysis, we estimated that the difference between the two tonometers decreased 0.7 mmHg per 10-μm increase in CCT and 0.3 mmHg per 1-mmHg increase in IOP value.
Scattergram showing the correlation between mean IOP readings obtained by dynamic contour tonometry (DCT) and Goldmann applanation tonometry (GAT) (mmHg) with increasing CCT (central corneal thickness) value (microns) in healthy subjects (a), patients with POAG (b), and patients with PACG (c). Solid line: linear function of the data for GAT. Broken line: linear function of the data for DCT.
Linear regression analysis showed no significant effects of CCT (P=0.43), CC (P=0.59), AL (P=0.41), or age (P=0.57) on DCT readings. On the contrary, IOP values obtained by GAT depended on CCT (P=0.001) but were not significantly influenced by CC (P=0.66), AL (P=0.77), or age (P=0.34).
The OPA values measured by DCT (range=1–7.9 mmHg) were significantly higher in the eyes with PACG than in either normal eyes (P<0.001) or in eyes with POAG (P<0.001) (Table 1). The linear regression analysis revealed that OPA readings were not affected by CCT (P=0.96), CC (P=0.19), or age (P=0.09), while the values were influenced by ΔIOP (P<0.001) and AL (P<0.001).
Discussion
In this study on healthy and glaucomatous subjects, 300 eyes from three different groups of Caucasian participants were accurately enrolled to create comparable samples (Table 1). We found a high correlation between DCT and GAT measurements and the agreement analysis revealed wide 95% limits of agreement between instruments (Figure 1). However, IOP readings obtained by DCT were 2.6 mmHg higher than those by GAT. Similar results, in spite of different mean values, have been reported by previous investigations.16, 19, 20, 21, 23, 24, 25, 26, 27, 28, 29, 30, 31, 35, 36 The ΔIOP between DCT and GAT readings was higher in healthy than in glaucomatous eyes, either with open-angle or angle-closure phenotypes (Table 1). The same outcome has been found by analysing IOP measurements obtained by DCT and GAT in different groups of subjects with the same CCT value, except for POAG subjects with thinner corneas. Furthermore, the significance of ΔIOP between healthy and glaucomatous eyes intensified with increasing CCT (Table 2). The explanation of this outcome was unclear. Topical IOP-lowering medications could bias the measurements of DCT on glaucomatous eyes. Both histological changes of collagen (due to an upregulation of matrix metalloproteinases) and modification of the corneal endothelial pump function have been reported after the administration of topical antiglaucoma therapy.37, 38 Drug-induced changes of corneal structural properties, such as hydration and rigidity, could explain the different ability of DCT compared to GAT to measure IOP in glaucomatous compared to healthy eyes. This observation requires further confirmation in healthy eyes subjected to manometry.
Several studies showed a significantly lower correlation of CCT with DCT than with GAT.16, 28, 29, 30, 31 Correspondingly, the present study indicated that DCT readings were statistically independent of CCT, CC, AL, or age. On the contrary, IOP values obtained by GAT depended on CCT but were not significantly influenced by other ocular structural factors and age.
Furthermore, the OPA readings were not influenced by CCT, CC, and age but were affected by ΔIOP and AL. Larger values of OPA were associated with a decrease in the difference between DCT and GAT and a decrease of AL values. These findings explain why OPA values were found to be higher in the glaucomatous patients with angle-closure phenotype (lowest AL) than either normal subjects (median AL) (P<0.001) or glaucomatous patients with open-angle phenotype (highest AL) (P<0.001). For eyes with low values of OPA, DCT measurements tended to be higher than GAT, whereas for eyes with high values of OPA, GAT measurements tended to be higher than DCT. While DCT provides a digital readout of the OPA, which represents the average of the minimum IOP readings during the cardiac cycle (diastolic phase), GAT readings were obtained during the systolic phase. These findings explain why the differences between DCT and GAT were related to OPA. Similar results, in spite of different study populations, have been reported by previous investigations.39, 40
Moreover, we evaluated the performance of DCT over a wide range of CCT and IOP values recorded in our study population. The ΔIOP between DCT and GAT measurements decreased with an increase in CCT (Figures 2 and 3) and IOP values (Figure 4). This finding is in agreement with other studies that showed that the difference between the tonometers declined as CCT increased.36, 41 On the contrary, only Barleon et al35 observed that the difference between the two instruments was greatest in eyes with lower IOP value, gradually lessening as IOP increased. Considering the values recorded in our limited study population, we found an inverse association between DCT and GAT for CCT values above 620 μm and IOP values above 24 mmHg. There is evidence of varying bias, as indicated by the slope of the regression line of the differences between the instruments: for smaller CCT and IOP values, DCT measurements tended to be higher than GAT measurements, whereas for higher CCT (>620 μm) and IOP values (>24 mmHg), DCT measurements tended to be lower than GAT measurements, whether in healthy or in glaucomatous subjects. The existence of varying bias indicates that the amount of disagreement between the two instruments is not constant throughout the range of measurements, but rather varies with the actual measurement.
In our study, the wide and varying 95% limits of agreement between DCT and GAT indicated that, although they are highly correlated, measurements should not be used interchangeably. Our study reveals a good overall correlation between DCT and GAT, but the high correlation of 0.93 masks the important systematic difference between these machines and the agreement between instruments differs in high or low IOP ranges. This difference between IOP values from DCT and GAT could be clinically significant and might alter management decisions in some cases.
References
Whitacre MM, Stein R . Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol 1993; 38: 1–30.
Inoue K, Okugawa K, Oshika T, Amano S . Morphological study of corneal endothelium and corneal thickness in pseudoexfoliation syndrome. Jpn J Ophthalmol 2003; 47: 235–239.
Whitacre MM, Stein RA, Hassanein K . The effect of corneal thickness on applanation tonometry. Am J Ophthalmol 1993; 115: 592–596.
Zadok D, Tran DB, Twa M, Carpenter M, Schanzlin DJ . Pneumotonometry versus Goldmann tonomerty after laser in situ keratomileusis for myopia. J Cataract Refract Surg 1999; 25: 1344–1348.
Mardelli PG, Piebenga LW, Whitacre MM, Siegmund KD . The effect of excimer laser photorefractive keratectomy on intraocular pressure measurements using the Goldmann applanation tonometer. Ophthalmology 1997; 104: 945–948.
Park HJ, Uhm KB, Hong C . Reduction in intraocular pressure after laser in situ keratomileusis. J Cataract Refract Surg 2001; 27: 303–309.
Rashad KM, Bahnassy AA . Changes in intraocular pressure after laser in situ keratomileusis. J Refract Surg 2001; 17: 420–427.
Duch S, Serra A, Castanera J, Abos R, Quintana M . Tonometry after laser in situ keratomileusis treatment. J Glaucoma 2001; 10: 261–265.
Fournier AV, Podtetenev M, Lemire J, Thompson P, Duchesne R, Perreault C et al. Intraocular pressure change measured by Goldmann tonometry after laser in situ keratomileusis. J Cataract Refract Surg 1998; 24: 905–910.
Abbasoğlu ÖE, Bowman RW, Cavanagh HD, Cavanagh HD, McCulley JP . Reliability of intraocular pressure measurements after myopic excimer photorefractive keratectomy. Ophthalmology 1998; 105: 2193–2196.
Garzozi HJ, Chung HS, Yang Y, Kagemann L, Harris A . Intraocular pressure and photorefractive keratectomy; a comparison of three different tonometers. Cornea 2001; 20: 33–36.
Shah S, Chatterjee A, Mathai M, Kelly SP, Kwartz J, Henson D et al. Relationship between corneal thickness and measured intraocular pressure in a general ophthalmology clinic. Ophthalmology 1999; 106: 2154–2160.
Morad Y, Sharon E, Hefetz L, Nemet P . Corneal thickness and curvature in normal-tension glaucoma. Am J Ophthalmol 1998; 76: 319–324.
Copt RP, Thomas R, Mermoud A . Corneal thickness in ocular hypertension, primary open-angle glaucoma, and normal tension glaucoma. Arch Ophthalmol 1999; 117: 14–16.
Brandt JD, Beiser JA, Kass MA, Gordon MO . Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology 2001; 108: 1779–1788.
Kniestedt C, Michelle N, Stamper RL . Dynamic contour tonometry: a comparative study on human cadaver eyes. Arch Ophthalmol 2004; 122: 1287–1293.
Kanngiesser HE, Robert YCE . Dynamic contour tonometry [ARVO abstract]. Invest Ophthalmol Vis Sci 2002; 43: E-Abstract 301.
Burvenich H, Burvenich E, Vincent C . Dynamic contour tonometry (DCT) versus non-contact tonometry (NCT): a comparison study. Bull Soc Belge Ophthalmol 2005; 298: 63–69.
Schneider E, Grehn F . Intraocular pressure measurement—comparison of dynamic contour tonometry and goldmann applanation tonometry. J Glaucoma 2006; 15: 2–6.
Ku JK, Danesh-Meyer HV, Craig JP, Gamble GD, McGhee CN . Comparison of intraocular pressure measured by Pascal dynamic contour tonometry and Goldmann applanation tonometry. Eye 2006; 20: 191–198.
Pache M, Wilmsmeyer S, Lautebach S, Funk J . Dynamic contour tonometry versus Goldmann applanation tonometry. A comparative study. Graefes Arch Clin Exp Ophthalmol 2005; 243: 763–767.
Hoffmann C, Bachmann LM, Thiel MA . Intraocular pressure using dynamic contour tonometry and contact lens tonometry. BMC Ophthalmol 2004; 4: 4.
Kniestedt C, Lin S, Choe J, Nee M, Bostrom A, Stürmer J et al. Correlation between intraocular pressure, central corneal thickness, stage of glaucoma, and demographic patient data: prospective analysis of biophysical parameters in tertiary glaucoma practice population. J Glaucoma 2006; 15: 91–97.
Kotecha A, White ET, Shewry JM, Garway-Heath DF . The relative effects of corneal thickness and age on Goldmann applanation tonometry and dynamic contour tonometry. Br J Ophthalmol 2005; 89: 1572–1575.
Kniestedt C, Lin S, Choe J, Bostrom A, Nee M, Stamper RL . Clinical comparison of contour and applanation tonometry and their relationship to pachymetry. Arch Ophthalmol 2005; 123: 1532–1537.
Doyle A, Lachkar Y . Comparison of dynamic contour tonometry with goldmann applanation tonometry over a wide range of central corneal thickness. J Glaucoma 2005; 14: 288–292.
Kniestedt C, Kanngiesser H, Stamper RL . Assessment of Pascal dynamic contour tonometer in monitoring IOP after LASIK. J Cataract Refract Surg 2005; 31: 458–459.
Duba I, Wirthlin AC . Dynamic contour tonometry for post-LASIK intraocular pressure measurements. Klin Monastbl Augenheilkd 2004; 221: 347–350.
Siganos DS, Papastergiou GI, Moedas C . Assessment of the Pascal dynamic contour tonometer in monitoring intraocular pressure in unoperated eyes and eyes after LASIK. J Cataract Refract Surg 2004; 30: 746–751.
Kaufmann C, Bachmann LM, Thiel MA . Intraocular pressure measurements using dynamic contour tonometry after laser in situ keratomileusis. Invest Ophthalmol Vis Sci 2003; 44: 3790–3794.
Kaufmann C, Bachmann LM, Thiel MA . Comparison of dynamic contour tonometry with goldmann applanation tonometry. Invest Ophthalmol Vis Sci 2004; 45: 3118–3121.
Medeiros FA, Sample PA, Weinreb RN . Comparison of dynamic contour tonometry and Goldmann applanation tonometry in Africa American Subjects. Ophthalmology 2007; 114: 658–665.
Chylack LT, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL et al. The lens opacities classification system III. Arch Ophthalmol 1993; 33: 153–159.
Bland JM, Altman DG . Measuring agreement in method comparison studies. Stat Methods Med Res 1999; 8: 135–160.
Barleon L, Hoffmann EM, Berres M, Pfeiffer N, Grus FH . Comparison of dynamic contour tonometry and Goldmann applanation tonometry in glaucoma patients and healthy subjects. Am J Ophthalmol 2006; 142: 583–590.
Francis BA, Hsieh A, Lai MY, Chopra V, Pena F, Azen S et al. Effects of corneal thickness, corneal curvature and intraocular pressure level on Goldmann applanation tonometry and dynamic contour tonometry. Ophthalmology 2007; 114: 20–26.
Viestenz A, Martus P, Schlotzer-Schrehardt U, Langenbucher A, Mardin CY . Impact of prostaglandin-F(2alpha)-analogues and carbonic anhydrase inhibitors on central corneal thickness—a cross-sectional study on 403 eyes. Klin Monatsnl Augenheilkd 2004; 221: 753–756.
Kaminski S, Hommer A, Koyuncu D, Biowski R, Barisani T, Baumgartner I . Influence of dorzolamide on corneal thickness, endothelial cell count and corneal sensibility. Acta Ophthalmol Scand 1998; 76: 78–79.
Punjabi OS, Ho HK, Kniestedt C, Bostrom AG, Stamper RL, Lin SC . Intraocular pressure and ocular pulse amplitude comparisons in different types of glaucoma using dynamic contour tonometry. Curr Eye Res 2006; 31: 851–862.
Kaufmann C, Bachmann LM, Robert YC, Thiel MA . Ocular pulse amplitude in healthy subjects as measured by dynamic contour tonometry. Arch Ophthalmol 2006; 124: 1104–1108.
Pache M, Wilmsmeyer S, Lautebach S, Funk J . Dynamic contour tonometry versus Goldmnann applanation tonometry: a comparative study. Graefes Arch Clin Exp Ophthalmol 2005; 243: 763–767.
Acknowledgements
Piero Ceruti has full access to all the data in the study and takes responsibility of the integrity of the data and the accuracy of the data analysis. We do not have financial interest in any aspect of this study or sources of support. We do not have any commercial or proprietary interest in the product or company. We did not receive payment as a consultant, reviewer, or evaluator.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceruti, P., Morbio, R., Marraffa, M. et al. Comparison of Goldmann applanation tonometry and dynamic contour tonometry in healthy and glaucomatous eyes. Eye 23, 262–269 (2009). https://doi.org/10.1038/sj.eye.6703102
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6703102
Keywords
This article is cited by
-
Analysis of differences in intraocular pressure evaluation performed with contact and non-contact devices
BMC Ophthalmology (2018)
-
Evaluation von Korrekturformeln für die Tonometrie
Der Ophthalmologe (2017)
-
Clinical utility of spectral analysis of intraocular pressure pulse wave
BMC Ophthalmology (2014)
-
A comparison between Goldmann applanation tonometry and dynamic contour tonometry after photorefractive keratectomy
Graefe's Archive for Clinical and Experimental Ophthalmology (2013)