Abstract
Aim
To describe the anatomical and visual outcome of subfoveal and juxtafoveal choroidal neovascularization (CNV) in highly myopic eyes treated by intravitreal bevacizumab.
Methods
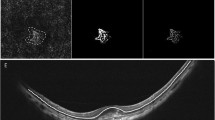
Prospective, nonrandomized, multicentric, interventional pilot study. Twenty-six highly myopic eyes from 25 patients with subfoveal and juxtafoveal CNV were treated by three monthly intravitreal injections with 1.25 mg bevacizumab. Patients were evaluated for best-corrected visual acuity (BCVA) and optical coherence tomography at baseline and then monthly. Fluorescein angiography was performed at baseline and at month 3.
Results
Patients averaged 49.5 years of age (SD 16.0, range 29–82). Five patients were male and 20 were female. BCVA at baseline averaged 20/62 (range 20/200–20/32) and 20/38 (range 20/160–20/20) at month 6. Average central foveal thickness was 282.4 μm (SD 68.3, range 168–447) at baseline and 224.0 μm (SD 46, range 132–294) at month 6. Fifteen eyes were naïve for treatment and 11 eyes had been previously treated by photodynamic therapy (PDT) (average 2.5 PDT sessions). Leakage from CNV had ceased in all eyes at month 3 and CNV was still closed at month 6. Neither ocular nor systemic safety issues appeared during the follow-up.
Conclusions
Intravitreal bevacizumab seems to be an effective and safe therapeutic procedure to treat subfoveal and juxtafoveal CNV in highly myopic eyes. Further studies are required to verify the efficacy and usefulness of this therapy compared with established treatments for this condition.
Similar content being viewed by others
Introduction
High myopia affects approximately 2% of global population. Myopic maculopathy is the main cause of vision loss among highly myopic patients, and the leading aetiology of subfoveal choroidal neovascularization (CNV) among patients younger than 50 years of age.1
Different therapeutic approaches have been tried to treat myopic CNV, including argon laser photocoagulation,2, 3 surgical removal, and macular translocation,4, 5 and, more recently, photodynamic therapy (PDT) with verteporfin.6, 7, 8 Visual deterioration secondary to retinal pigment epithelium (RPE) and choriocapillaris atrophy, and the frequent need to repeat PDT sessions have lead to the association of intravitreal triamcinolone and PDT. This combined therapy reduces the number of PDT sessions, although it is associated with a high risk of glaucoma and cataracts and does not seem to improve final visual acuity.9, 10
New antiangiogenic drugs have been made available to treat subfoveal CNV secondary to high myopia, although there have not been too many reports yet.11, 12, 13, 14, 15, 16, 17, 18, 19, 20 The purpose of this pilot study is to evaluate the outcome of intravitreal bevacizumab to treat subfoveal and juxtafoveal CNV in highly myopic eyes.
Materials and methods
Multicentric, prospective, nonrandomized, interventional pilot study. Twenty-six eyes from 25 highly myopic patients with active subfoveal and juxtafoveal classic CNV were treated by three monthly intravitreal injections of 1.25 mg bevacizumab. Patients being treated by PDT were started on intravitreal bevacizumab if they were losing visual acuity or the CNV did not respond to PDT. Written informed consent was obtained from the patients prior to the procedure, as well as an individualized approval was obtained from the National Ministry of Health. The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki, and data gathering was performed after obtaining written informed consent.
Patients were informed about the off-label condition of this therapy, and fertile patients were also informed about the possible risks of pregnancy and in utero exposition. Fertile patients agreed to use two forms of contraception (barrier and hormonal methods) throughout the 3 months of injections and during the following 3 months. Snellen best-corrected visual acuity (BCVA) was determined at 4 m using standard ETDRS charts (Lighthouse, New York, NY, USA) by certified optometrists. A complete ocular examination, BCVA, and optical coherence tomography (OCT) were performed at the first visit and then monthly during the follow-up. Fluorescein angiography was performed at the initial visit and at month 3. At each visit, the patients were specifically asked for the appearance of systemic (medication changes, high blood pressure as measured by their general practicionist, signs of cerebrovascular accidents, myocardial infarctions, or ischemia), and ocular (pain, floaters, and reduced visual acuity) side effects.
Results
Patients averaged 49.5 years of age (SD 16.0, range 29–82). Out of the patients 20 were female (11 of them under 50 years of age) and 5 were male. Average spherical equivalent was −14.0 D (SE 3.8, range −7.0 to −22.0 D). Eleven eyes had undergone previous unsuccessful PDT to treat myopic CNV: one patient eight times, two patients four times, five patients twice, and three patients once (one of the latter was associated with intravitreal triamcinolone). The last PDT session had been performed 3 months prior to the first injection of bevacizumab in all cases. Demographics of the patients are shown in Table 1.
Follow-up was longer than 3 months in all the cases while longer than 6 months in 24 cases. Neither ocular nor systemic side effects appeared during the follow-up.
Mean initial BCVA was 20/62 (range 20/200–20/32) (57 letters, SD 12.3, range 34–75). Mean initial central foveal thickness (CFT) was 282.4 μm (SD 68.3, range 168–447).
BCVA averaged 20/39 (range 20/125–20/20) (67.3 letters, SD 12.5, range 45–85) and CFT was 214.7 μm (SD 51.4, range 117–312) at month 3 (P=0.0006 and P=0.0008, respectively; Student's t-test for paired data).
BCVA averaged 20/38 (range 20/160–20/20) (69 letters, SD 14.2, range 40–84) and CFT was 224.0 μm (SD 46, range 132–294) at month 6 (P=0.0034 and P=0.0002, respectively; Student's t-test for paired data).
BCVA was 20/40 or better in 16/26 eyes at month 3 and in 15/24 eyes at month 6. Twelve of 24 eyes with more than 6 months follow-up gained two or more ETDRS lines (10 or more letters), 10 remained within one line from baseline and two eyes lost 2 or more lines.
Discussion
Long-term visual outcome of myopic CNV seems to be extremely poor according to the reported series based on its natural history, suggesting that active treatments should be recommended to prevent long-term visual impairment.21 Yoshida et al21 have reported a natural history of visual acuity dropping from 70% of the eyes with 20/200 or better at the onset of CNV to 55% at 3 years and 11% at 5 years. The results of PDT do not seem to be entirely satisfactory, as has been previously reported by our group, with 28% of the patients younger than 55 years and 54% of those older than 55 years, losing two or more lines by the end of the first year of treatment.7
The presence of high levels of vascular endothelial growth factor and pigment epithelium-derived factor is suspected to be involved in the development of myopic CNV.22 Antiangiogenic therapies (isolated or in association with PDT) have been used to treat subfoveal myopic CNV such as intravitreous9, 10, 23, 24 triamcinolone or intravitreous bevacizumab.11, 12, 13, 14, 15, 16, 17, 18, 19, 20 To our knowledge, no reports on the use of pegaptanib or ranibizumab (the only approved drugs for intravitreal use) have been published and no clinical trials are underway.
Tewari et al,17 Laud et al,19 and Nguyen et al20 have reported the utility of intravitreal bevacizumab in CNV associated with high myopia. Yamamoto et al11 have recently published a longer series consisting 11 eyes from nine patients. They treated 11 eyes (5 of them were previously unsuccessfully treated by PDT) by one or two intravitreal injections of 1.25 mg bevacizumab with an average 5 months follow-up. In their series, 8/11 eyes ended with BCVA better than 20/50. Similarly, Sakaguchi et al injected 1 mg bevacizumab in eight highly myopic eyes with CNV. BCVA improved in 6/8 eyes, remaining stable in two cases after at least 3 months follow-up, while OCT retinal thickness decreased.12 Both results were statistically significant.
More recently, Mandal et al13 and Chan et al15 have reported their results on myopic patients treated by intravitreal bevacizumab and followed for 5 to 6 months. Chan et al performed three monthly intravitreal injections of bevacizumab followed further by monthly injections in those cases where CNV was not closed. Out of those cases, 90% of the eyes showed angiographic closure by month 3 with an average gain of 13 letters by month 6. Mandal et al performed repeated bevacizumab injections in those cases where OCT showed intraretinal oedema, subretinal fluid, and/or pigment epithelial detachment, with an average gain of more than 20 letters by month 6. Both studies showed significant reduction in macular thickness. These results are similar to ours with 12 letters gained, final BCVA better than 20/40 in 15/24 eyes with more than 6 months follow-up, and significant reduction in macular thickness. The better outcome of the patients reported by Chan et al and Mandal et al may be secondary to the previous PDT sessions performed in 11/26 eyes in our series, which are known to induce RPE and choriocapillaris atrophy.25
The number of intravitreal injections needed to achieve CNV closure in highly myopic eyes is not known. Several different protocols are presently being used with antiangiogenic drugs as has been reported for the PIER, PrONTO, and SAILOR trials with ranibizumab. The results from the PrONTO study may imply that less frequent injections adjusted by OCT follow-up may be enough.26 However, accurate determinations of CNV activity may occasionally be difficult in highly myopic eyes. We have performed three consecutive bevacizumab injections to achieve complete CNV inactivation and prevent recurrences.
None of the mentioned authors have reported adverse side effects associated with intravitreal bevacizumab.11, 12, 13, 14, 15, 17, 19, 20 Lynch and Cheng27 performed a revision on 7113 injections of bevacizumab with less than 0.21% adverse events. In our series, we have found that four patients had mild loss in BCVA, which was attributed to progression of macular atrophy secondary to high myopia. Three of these cases had decreased retinal thickness, while one did not show any changes after the injections. It is remarkable that the eye with unchanged retinal thickness had previously undergone unsuccessful PDT associated with intravitreous triamcinolone and one further eye with decreased BCVA had also undergone four PDT sessions. However, previous PDT does not seem to be a predictor of poor results of intravitreal bevacizumab as three of six eyes treated by PDT improved BCVA.
The use of intravitreal antiangiogenic drugs to treat myopic CNV faces some specific risks, in addition to those inherent to any intravitreous injection. First of all, the higher frequency of degenerative lesions and peripheral vitreoretinal adhesions may increase the chances for retinal tears or detachments associated with the procedure. Secondly, the use of antiangiogenic drugs among younger patients, many of them on fertile age, increases the concern on teratogenic effects. On the other hand, the advantages of this therapy compared with intravitreous triamcinolone associated with PDT are a lower risk of cataracts and glaucoma, as well as less atrophic changes on the macular RPE.
Intravitreous bevacizumab seems to be a useful procedure to treat CNV associated with high myopia, as a first line therapy and after unsuccessful previous PDT. Special care should be taken with highly myopic patients with peripheral retinal degenerations predisposing to retinal tears and detachments, as well as with fertile patients.
Randomized clinical trials comparing this therapy with PDT and longer follow-up studies are needed to evaluate the real efficacy of this therapy and the safest injection regime.
References
Cohen SY, Laroche A, Leguen Y, Soubrane G, Coscas GJ . Etiology of choroidal neovascularization in young patients. Ophthalmology 1996; 103: 1241–1244.
Ruiz-Moreno JM, Montero JA . Long-term visual acuity after argon green laser photocoagulation of juxtafoveal choroidal neovascularization in highly myopic eyes. Eur J Ophthalmol 2002; 12: 117–122.
Secretan M, Kuhn D, Soubrane G, Coscas G . Long-term visual outcome of choroidal neovascularization in pathologic myopia: natural history and laser treatment. Eur J Ophthalmol 1997; 7: 307–316.
Cekic O, Ohji M, Fujikado T, Fang XY, Hayashi A, Kusaka S et al. Foveal translocation surgery and myopic subfoveal CNV membrane. Ophthalmology 2000; 107: 2117.
Uemura A, Thomas MA . Subretinal surgery for choroidal neovascularization in patients with high myopia. Arch Ophthalmol 2000; 118: 344–350.
Verteporfin in Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin 1-year results of a randomized clinical trial--VIP report no. 1. Ophthalmology 2001; 108: 841–852.
Montero JA, Ruiz-Moreno JM . Verteporfin photodynamic therapy in highly myopic subfoveal choroidal neovascularisation. Br J Ophthalmol 2003; 87: 173–176.
Ruiz-Moreno JM, Montero JA . Subretinal fibrosis after photodynamic therapy in subfoveal choroidal neovascularisation in highly myopic eyes. Br J Ophthalmol 2003; 87: 856–859.
Chan WM, Lai TY, Wong AL, Liu DT, Lam DS . Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of choroidal neovascularisation secondary to pathological myopia: a pilot study. Br J Ophthalmol 2007; 91: 174–179.
Montero JA, Ruiz-Moreno JM . Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of choroidal neovascularisation secondary to pathological myopia: a pilot study. Br J Ophthalmol 2007; 91: 131–133.
Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS . Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathological myopia. Br J Ophthalmol 2007; 91: 157–160.
Sakaguchi H, Ikuno Y, Gomi F, Kamei M, Sawa M, Tsujikawa M et al. Intravitreal injection of bevacizumab for choroidal neovascularisation associated with pathological myopia. Br J Ophthalmol 2007; 91: 161–165.
Mandal S, Venkatesh P, Sampangi R, Garg S . Intravitreal bevacizumab (Avastin) as primary treatment for myopic choroidal neovascularization. Eur J Ophthalmol 2007; 17: 620–626.
Hernandez-Rojas ML, Quiroz-Mercado H, Dalma-Weiszhausz J, Fromow-Guerra J, Amaya-Espinosa A, Solis-Vivanco A et al. Short-term effects of intravitreal bevacizumab for subfoveal choroidal neovascularization in pathologic myopia. Retina 2007; 27: 707–712.
Chan WM, Lai TY, Liu DT, Lam DS . Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization six-month results of a prospective pilot study. Ophthalmology 2007; June 26; [e-pub ahead of print].
Yamamoto I, Rogers AH, Reichel E, Yates P, Duker JS . Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularization secondary to pathologic myopia. Br J Ophthalmol 2007; 91: 157–160.
Tewari A, Dhalla MS, Apte RS . Intravitreal bevacizumab for treatment of choroidal neovascularization in pathologic myopia. Retina 2006; 26: 1093–1094.
Sakaguchi H, Ikuno Y, Gomi F, Kamei M, Sawa M, Tsujikawa M et al. Intravitreal injection of bevacizumab for choroidal neovascularization caused by pathological myopia. Br J Ophthalmol 2007; 91: 161–165.
Laud K, Spaide RF, Freund KB, Slakter J, Klancnik JM, Jr . Treatment of choroidal neovascularization in pathologic myopia with intravitreal bevacizumab. Retina 2006; 26: 960–963.
Nguyen QD, Shah S, Tatlipinar S, Do DV, Anden EV, Campochiaro PA . Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol 2005; 89: 1368–1370.
Yoshida T, Ohno-Matsui K, Yasuzumi K, Kojima A, Shimada N, Futagami S et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology 2003; 110: 1297–1305.
Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol 2006; 141: 456–462.
Degenring RF, Jonas JB . Photodynamic therapy in combination with intravitreal triamcinolone for myopic choroidal neovascularization. Acta Ophthalmol Scand 2005; 83: 621.
Marticorena J, Gomez-Ulla F, Fernandez M, Pazos B, Rodriguez-Cid MJ, Sanchez-Salorio M . Combined photodynamic therapy and intravitreal triamcinolone acetonide for the treatment of myopic subfoveal choroidal neovascularization. Am J Ophthalmol 2006; 142: 335–337.
Wachtlin J, Behme T, Heimann H, Kellner U, Foerster MH . Concentric retinal pigment epithelium atrophy after a single photodynamic therapy. Graefes Arch Clin Exp Ophthalmol 2003; 241: 518–521.
Rosenfeld PJ, Rich RM, Lalwani GA . Ranibizumab: phase III clinical trial results. Ophthalmol Clin North Am 2006; 19: 361–372.
Lynch SS, Cheng CM . Bevacizumab for neovascular ocular diseases. Ann Pharmacother 2007; 41: 614–625.
Acknowledgements
Written informed consent and individualized approval from the National Ministry of Health was obtained prior to the procedure. This study has been performed in accordance with the ethical standards of the 1964 Declaration of Helsinki, and data gathering was performed after obtaining written informed consent. Patients were informed about the off-label situation of this therapy and fertile patients were also informed about the possible risks of pregnancy and in utero exposition.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have neither proprietary nor financial interest in the devices and drugs described in this paper.
Rights and permissions
About this article
Cite this article
Ruiz-Moreno, J., Gomez-Ulla, F., Montero, J. et al. Intravitreous bevacizumab to treat subfoveal choroidal neovascularization in highly myopic eyes: short-term results. Eye 23, 334–338 (2009). https://doi.org/10.1038/sj.eye.6703052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6703052
Keywords
This article is cited by
-
Management of Myopic Choroidal Neovascularization: Focus on Anti-VEGF Therapy
Drugs (2016)
-
Comparison of 1-year therapeutic effect of ranibizumab and bevacizumab for myopic choroidal neovascularization: a retrospective, multicenter, comparative study
BMC Ophthalmology (2014)
-
Summary of prognostic factors for choroidal neovascularization due to pathological myopia treated by intravitreal bevacizumab injection
Graefe's Archive for Clinical and Experimental Ophthalmology (2012)
-
Myopic choroidal neovascularization treated by intravitreal bevacizumab: comparison of two different initial doses
Graefe's Archive for Clinical and Experimental Ophthalmology (2011)
-
Intravitreal bevacizumab for choroidal neovascularisation secondary to causes other than age-related macular degeneration
Eye (2010)