Abstract
Purpose
There have been few viable alternatives to patching the better eye as a treatment of amblyopia for more than two centuries. The success of patching depends on compliance, which is problematic for up to 59% of children and their families.
Methods
This pilot study trialled the interactive binocular treatment (I-BiT) system as an alternative amblyopia treatment in 12 older amblyopes (6.1–11.4 years, median 8.2), who had not complied with or responded to occlusion. Virtual reality images were projected to each eye simultaneously via a headset during eight treatment sessions of 25-min duration. Outcome measures were changes in high- (HCVA) and low-contrast log MAR acuity (LCVA) at 1 week, 4 weeks and a final follow-up (3–18 months) after the final treatment.
Results
Sustained improvements in HCVA were observed in seven children (58%) and in LCVA in eight children (67%), including two for whom amblyopia was eliminated. Five children had visual acuities equivalent to 6/12 or better at least 6 months after stopping treatment, compared with one child prior to treatment. Significant improvements in HCVA occurred up to the fourth treatment; in LCVA to the seventh treatment.
Conclusion
Sustained improvements in visual acuity were observed for 58% of this small group of children using the I-BiT system, despite prior failure with conventional treatment. This offers hope for a potential time-saving alternative to patching, in which compliance can easily be monitored, but the results need to be validated by means of a randomised controlled trial.
Similar content being viewed by others
Introduction
Amblyopia, impaired vision in one or both eyes, affects up to 5% of the population.1 Patching of the eye with better vision is the principal treatment for unilateral amblyopia, combined with full-time wear of any appropriate spectacle correction.2, 3 Compliance with patching, which is essential to success,4, 5, 6, 7 has proven problematic for up to 59% of children and their families.8, 9, 10, 11, 12, 13, 14 Patching can cause social stigma, and have a detrimental impact on family, school, and social life.12, 15, 16 Although most of the treatment effect probably occurs within the first 120–200 h or 6 weeks of patch wear,17, 18 in normal clinical practice, patching can continue for many months or years,17, 19 placing considerable demands on the child and family.
A computer-based virtual reality system (interactive binocular treatment (I-BiT) system) has been developed by a multidisciplinary group from the Queens Medical Centre in Nottingham and the University of Nottingham,20 and piloted on amblyopes in both Nottingham21 and Glasgow. The results for the Glasgow Pilot Study are reported here. The study aimed to assess the effect of this treatment on high- (HCVA) and low-contrast visual acuity (LCVA) in older amblyopes who had failed to respond to or comply with occlusion.
Methods
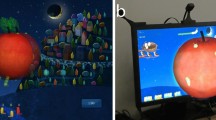
The technical details of the computer-based treatment system (the I-BiT system) and the stimulus used in the current study are reported elsewhere.20 Virtual reality invokes a three-dimensional image in those with normal binocular single vision (BSV) by stimulating both eyes simultaneously. The Nottingham study20 included children with abnormal BSV and suppression, and all of these perceived the images projected to both eyes, but it is not known whether images were perceived in depth. The only technical difference for the current study was in delivering the binocular stimulus via a V8 head-mounted display from Virtual Research (Figure 1). This allowed inter-pupillary distance (IPD) adjustment during calibration. Calibration confirms superimposition and sensory fusion using images projected to either eye, similar to those used with the synoptophore.
Children attended 11 weekly sessions (Table 1): eight ‘treatment’ and three ‘assessment’ only, with subsequent follow-up 1 and 4 weeks after stopping treatment, with an additional final follow-up (range: 3–18 months, median 8 months).
Treatment composed of two components:
-
i
Twenty minutes watching a video-clip, the detail being viewed by the amblyopic eye. The fellow eye viewed the surrounding frame, which incorporated four individually coloured squares at the top, bottom, left, and right of the screen, whose colour changed at regular intervals. The examiner asked the child to report the colour changes of the four dots as they occurred, to check that both eyes perceived the images simultaneously.
-
ii
Five minutes playing an interactive driving game, with the detail of the visual scene split between the two eyes. The child drove the car around the circuit using a joystick, and was encouraged to pick up ‘tokens’, half presented to the amblyopic eye and half to the fellow eye. Superimposition of the images to the two eyes is necessary to appreciate the entire visual scene of the game, and on questioning, this was the case for all of the current cohort. If one eye is suppressed, part of the visual scene projected to the suppressed eye would be missing, and the child would not be able to drive the car around the track with any accuracy. All children in the current study also selected tokens presented to either eye. This confirms binocular vision, at least in its most basic form, that is the two eyes perceived the visual scene. Binocular single vision is assumed since children described a complete visual scene.
High- and low-contrast log MAR visual acuity (VA) were assessed with the log MAR crowded test22 before and after each treatment session. The low-contrast version had a contrast level of 21%. Binocularity/suppression was assessed to monitor the risk of diplopia prior to each treatment. This was achieved by means of Bagolini glasses, measurement of the fusion range for those with BSV, or assessment with the Sbisa bar for those with suppression. Where suppression density fell below the arbitrary level of 10, treatment was stopped. This occurred for three children.
Assessment sessions comprised of a detailed orthoptic assessment, which included VA assessment, cover test, assessment of BSV/suppression, including density of suppression with the Sbisa bar, and fixation using an ophthalmoscope with a fixation graticule.
Inclusion criteria
Children were recruited from the Orthoptic Department at Gartnavel General Hospital, all were registered under the care of one consultant paediatric ophthalmologist GN Dutton. Written parental consent was a pre-requisite of enrollment. The study was according to the Helsinki Declaration II for human experimentation and approved by the Hospital Research Ethics Committees.
The criteria for inclusion in the study were:
-
Non-organic amblyopia, pathology having been excluded by a detailed ophthalmic investigation, which included dilated ophthalmoscopy.
-
Full cycloplegic spectacle correction worn full-time for ⩾18 weeks,3 with last cycloplegic refraction within the last 12 months.
-
An interocular acuity difference of 0.2 log units (eight letters) or greater, assessed with the log MAR crowded test. Amblyopia is defined as an interocular difference of at least 0.1 log units (four letters) with this test.23
-
Prior failure to respond to or comply with occlusion, but no occlusion attempted in the past 3 months.
-
Age ⩾6 years to ensure co-operation with the treatment and detailed visual assessment.
-
stable VA over a minimum of the past two visits, consistent with the repeatability of the log MAR crowded test.24
Outcome measures
These followed two of the recommendations of the monitored occlusion treatment in amblyopia study group.25
-
1
High and low-contrast VA for the amblyopic eyes at 1 and 4 weeks and HCVA at a final visit after stopping treatment. A change in log MAR VA of 0.125 log units (five letters) is consistent with a treatment effect for this test.24
-
2
Interocular acuity difference that represents the amount of residual amblyopia.
The VA data were analysed using non-parametric statistics (Wilcoxon signed-rank test (WSRT)) as the data were paired and did not follow a normal distribution. Median values are quoted due to the small group size.
Study group
The study group comprised 12 children, 6 females and 6 males, ranging from 6.1 to 11.4 years (median 8.2). Five of them had failed to comply with prescribed occlusion and the other seven failed to respond to occlusion. Their details are shown in Table 2.
The amblyopia category was either strabismic (n=5) or mixed, that is a combination of anisometropic and strabismic (n=7). Starting VA for the amblyopic eyes ranged from 0.350 to 0.875 log units with interocular difference of 0.3–1.0 log units (12–40 letters).
Results
Amblyopic eye VA
By 1 week after stopping treatment (visit 11), HCVA had improved in 9 of 12 amblyopic eyes (75%) (Figure 2, range: 0.125–0.35 log units), WSRT Z corrected for ties −3.06, P=0.002. For two of these children, amblyopia was eliminated, based on both uniocular VA and interocular acuity difference (<0.1 log units, ie <4 letters). Changes for the fellow eyes are shown in Figure 3 for comparison. LCVA had improved in 8 of 12 amblyopic eyes (67%) (Figure 4, range: 0.125–0.375 log units; WSRT: Z corrected for ties −2.74, P=0.006). The changes for the fellow eye are shown in Figure 5.
By 4 weeks post-treatment, there was some regression of HCVA for one child and of LCVA for two children (WSRT: Z corrected for ties −2.747, P=0.006), but late improvements were noted for others: 1 HCVA by 0.3 log units (12 letters); 2 LCVA by 0.125 (five letters) and 0.175 log units (seven letters) respectively.
At the final follow-up, seven children (58%) maintained their best HCVA level (WSRT: Z corrected for ties −2.99, P=0.028), and eight (67%) their best LCVA (WSRT: Z corrected for ties −2.49, P=0.012).
Residual amblyopia
HCVA
By 1 week post-treatment, residual amblyopia ranged from 2 to 34 letters (mean 16, SD 11.6), which was significantly smaller than the interocular difference pre-treatment (range: 12–40 letters, mean 21.5, SD 8.9), WSRT Z corrected for ties −2.35, P=0.019. By 4 weeks post-treatment, although the mean remained reduced (range: 2–35 letters, mean 17.6, SD 11), the difference was no longer significant (P=0.059).
By the time of the final visit, residual amblyopia ranged from 1 to 32 letters (mean 16.8, SD 10.5), P=0.116.
LCVA
Residual amblyopia 1-week post-treatment had the same range, mean and SD as for HCVA. This was however, significantly different to the pre-treatment values (range: 7–45 letters, mean 19, SD 12; WSRT: Z corrected for ties −1.96, P=0.05). There were no significant changes at week 4 or at the final visit (P>0.25).
The number of treatments to achieve best HCVA and LCVA varied from 2 to 8, with improvement tending to be slightly slower for LCVA (median 7; HCVA median 5).
Prior to treatment, clinical testing confirmed abnormal binocular single vision (ABSV) in eight children and suppression in the other four children (Table 3). In seven children, the diagnosis was microtropia, a diagnostic feature of which is ABSV. The other child with ABSV had a deviation of 16 prism dioptres (pd) at near and 10 pd at distance. Suppression density reduced transiently to below filter 10 in three children (nos. 2, 4, 8) during the initial treatment block, necessitating ending the treatment. One of these (no. 4) was resumed after two visits, once suppression density had increased again to an acceptable level. Following treatment, one patient converted from microtropia with ABSV to normal binocular single vision (NBSV) (no. 5). In others, binocularity remained unchanged.
For those with BSV prior to treatment (n=8), there were significant changes in near or distance fusion ranges. The median value for near fusion range increased from 24 (range: 14–41) to 29.5 pd (range: 14–48), and for the distance range from 12 (range: 2–32) to 20 pd (range: 8–36). For four individuals, there was an increase in range of more than 10 pd.
The median stereoacuity value reduced from 400 (range: 1980–110) to 200 s of arc (range: 1980–110). Individual changes can be seen in Table 3.
Three of the four children with suppression on clinical testing showed sustained improvements in HCVA (nos. 2, 4, 8), compared with four of the eight with ABSV (nos. 1, 5, 9, 11).
Anecdotally, most children commented that they enjoyed the treatment and in those who improved, parents commented that there had been an improvement in performance at school, and reading abilities. Weekly attendance did not prove a problem to any of the parents.
Discussion
Our findings accord with those of the Nottingham group pilot study.21 Seven of 12 children (58%) achieved sustained improvements in high- and low-contrast VA in response to treatment using the I-BiT system, and for two children (17%; both with mixed amblyopia), amblyopia was eliminated. Although VA changes were modest, they occurred for a group of children for whom conventional treatment had failed and who were outwith the optimal period of visual plasticity. Mean and maximum improvements in high-contrast VA at the end of treatment were smaller for our group (mean 0.177 log units; maximum 0.350) than for the Nottingham pilot (0.271 log units; 0.550). Age differences could be the reason since all of our subjects were over 6 years, compared with only two of the six Nottingham children. Age clearly does not, however, preclude treatment benefits, since those with sustained improvements in vision ranged from 7.3 to 11.4 years, and occlusion success in older children/adolescents is well documented.26, 27, 28, 29, 30
Statistical significance disappeared after 1 week post-treatment when assessing residual amblyopia vs the amblyopic eye outcome alone. This reflects not only regression for some amblyopic eyes, but also improvement for some fellow eyes. This highlights the importance of reporting results for both eyes.
How do the results compare to occlusion? Recent studies of new amblyopes have found average improvements of 0.24–0.58 log units with occlusion worn over a maximum of 4 months for children up to 7 years.6, 7, 31, 32, 33 These are comparable to the Nottingham pilot study.
Although for most children, both low- and high-contrast VA improved, the amount and rate of improvement were rarely concordant. Low-contrast VA tended to change more slowly than HCVA, which is similar to the findings of an occlusion study.34
This new treatment offers hope for an enjoyable alternative to occlusion for many children. The time course of treatment effect for the I-BiT system offers great advantages over occlusion (100 min of I-BiT over ⩽8 weeks vs a minimum of 120–200 h of occlusion over several months), although this needs to be defined in a larger group. A reduction in the number of hospital appointments over which treatment is undertaken has potential cost benefits to the National Health Service, which could be balanced against the cost of the equipment. It removes the compliance issue at home/school by undertaking treatment on an out-patient basis. It also avoids the costly practice of admitting persistent non-compliers to hospital for occlusion enforcement.35
The system is not risk-free. As with occlusion in older children, the risk of overcoming suppression, resulting in diplopia, must be monitored closely using a Sbisa bar. This is illustrated in the three children in our study who manifested a temporary reduction in depth of suppression, but none complained of diplopia at any time.
The theoretical implications are intriguing, since the putative underlying mechanism of the treatment differs from conventional occlusion by maintaining and encouraging binocularity, and by including a hand–eye coordination task. Since the treatment involves binocular stimulation, an effect on BSV might be expected. One child did convert from ABSV with microtropia to NBSV, and some individual improvements in fusion range and stereoacuity were observed. To test the significance of any treatment effect on BSV, a stringent protocol is necessary, as well as normative data on the repeatability of fusion and stereoacuity measurements. Sustained improvements in VA were not confined to those with BSV on clinical testing. Adjustment of the IPD as part of the equipment set-up, is likely to have favoured BSV. In effect, this corrected for the subjective angle of deviation in a similar way to the synoptophore, so stimulated corresponding retinal areas, and moved the image viewed by the amblyopic eye outwith any suppression area. The large field stimulus is also likely to have been advantageous to peripheral fusion, in line with the finding that retinal correspondence may remain normal in the peripheral field, while abnormal retinal correspondence co-exists in the central field.36
The motion of the stimulus may also play a role in the therapeutic effect, by stimulating V1 via the magnocellular pathway.37
A randomised masked controlled trial is now warranted, applying the treatment to previously untreated children, and further studies are needed to understand what aspects of the treatment work.
Conflict of interest
Int. Pat. App. WO 03/092482. Technology licensed to Carlton Optical Research Ltd. One of the authors, Amanda Moody, is listed as one of the inventors in the patent and therefore has a commercial interest in the product, in conjunction with the University of Nottingham and Queens Medical Centre. All other authors have no commercial interest in the system.
References
Attebo K, Mitchell P, Cumming R, Smith W, Jolly N, Sparkes R . Prevalence and causes of amblyopia in an adult population. Ophthalmology 1998; 105: 154–159.
Royal College of Ophthalmology guidelines on strabismus and amblyopia 2006. http://www.rcophth.ac.uk/docs/scientific/ListofPublished&PendingGuidelinesJanuary2006.pdf.
Stewart CE, Moseley MJ, Fielder AR, Stephens DA, MOTAS Cooperative. Refractive adaptation in amblyopia: quantification of effect and implications for practice. Br J Ophthalmol 2004; 88(12): 1552–1556.
Newsham D . Parental non-concordance with occlusion therapy. Br J Ophthalmol 2000; 84(9): 957–962.
Loudon SE, Polling JR, Simonsz HJ . A preliminary report about the relation between visual acuity increase and compliance in patching therapy for amblyopia. Strabismus 2002; 10(2): 79–82.
Awan M, Proudlock FA, Gottlob I . A randomized controlled trial of unilateral strabismic and mixed amblyopia using occlusion dose monitors to record compliance. Invest Ophthalmol Vis Sci 2005; 46(4): 1435–1439.
Stewart CE, Fielder AR, Stephens DA, Moseley MJ . Treatment of unilateral amblyopia: factors influencing visual outcome. Invest Ophthalmol Vis Sci 2005; 46(9): 3152–3160.
Smith LK, Thompson JR, Woodruff G, Hiscox F . Factors affecting treatment compliance in amblyopia. J Pediatr Ophthalmol Strabismus 1995; 32(2): 98–101.
Foley-Nolan A, McCann A, O'Keefe M . Atropine penalisation versus occlusion as a primary treatment for amblyopia. Br J Ophthalmol 1997; 81: 54–57.
Beardsell R, Clarke S, Hill M . Outcome of occlusion treatment for amblyopia. J Pediatr Ophthalmol Strabismus 1999; 36(1): 19–24.
Oto S, Pelit A, Aydin P . Non-concordance in amblyopia treatment: the effective use of ‘smileys’. Strabismus 2002; 10(1): 23–30.
Searle A, Norman P, Harrad R, Vedhara K . Psychosocial and clinical determinants of compliance with occlusion therapy for amblyopic children. Eye 2002; 16(2): 150–155.
Loudon SE, Polling JR, Simonsz HJ . Electronically measured compliance with occlusion therapy for amblyopia is related to visual acuity increase. Graefes Arch Clin Exp Ophthalmol 2003; 241(3): 176–180.
Chua B, Johnson K, Martin F . A retrospective review of the associations between amblyopia type, patient age, treatment compliance and referral patterns. Clin Experiment Ophthalmol 2004; 32(2): 175–179.
Packwood EA, Cruz OA, Rychwalski PJ, Keech RV . The psychosocial effects of amblyopia study. J AAPOS 1999; 3(1): 15–17.
Cole SR, Beck RW, Moke PS, Celano MP, Drews CD, Repka MX, et al., Pediatric eye disease investigator group. The amblyopia treatment index. J AAPOS 2001; 5(4): 250–254.
Cleary M . Efficacy of occlusion for strabismic amblyopia: can an optimal duration be identified? Br J Ophthalmol 2000; 82(6): 572–578.
Stewart CE, Moseley MJ, Stephens DA, Fielder AR . Treatment dose–response in amblyopia therapy: the monitored occlusion treatment of amblyopia study (MOTAS). Invest Ophthalmol Vis Sci 2004; 45(9): 3048–3054.
Tan JH, Thompson JR, Gottlob I . Differences in the management of amblyopia between European countries. Br J Ophthalmol 2003; 87(3): 291–296.
Eastgate RM, Griffiths GD, Waddingham PE, Moody AD, Butler TK, Cobb SV et al. Modified virtual reality technology for treatment of amblyopia. Eye 2006; 20(3): 370–374.
Waddingham PE, Butler TK, Cobb SV, Moody AD, Comaish IF, Haworth SM et al. Preliminary results from the use of the novel interactive binocular treatment (I-BiTtrade mark) system, in the treatment of strabismic and anisometropic amblyopia. Eye 2006; 20(3): 375–378.
McGraw PV, Winn B . Glasgow Acuity Cards: a new test for the measurement of letter acuity. Ophthalmic Physiol Opt 1993; 13: 400–404.
Simmers AJ, Gray LS, Spowart K . Screening for amblyopia: a comparison of paediatric letter tests. Br J Ophthalmol 1997; 81(6): 465–469.
McGraw PV, Winn B, Gray LS, Elliott DB . Improving the reliability of visual acuity measures in young children. Ophthalmic Physiol Opt 2000; 20: 173–184.
Stewart CE, Moseley MJ, Fielder AR . Defining and measuring treatment outcome in unilateral amblyopia. Br J Ophthalmol 2003; 87(10): 1229–1231.
Mintz-Hittner HA, Fernandez KM . Successful amblyopia therapy initiated after age 7 years compliance cures. Arch Ophthalmol 2000; 118: 1535–1541.
Mohan K, Kanwar MS, Saroha V, Vandana MS, Sharma A, Ashok MS . Successful occlusion therapy for amblyopia in 11–15-year-old children. J Pediatr Ophthalmol Strabismus 2004; 41(2): 89–95.
Park KH, Hwang JM, Ahn JK . Efficacy of amblyopia therapy initiated after 9 years of age. Eye 2004; 18(6): 571–574.
Pediatric Eye Disease Investigator Group. A prospective, pilot study of treatment of amblyopia in children 10 to <18 years old. Am J Ophthalmol 2004; 137(3): 581–583.
Pediatric Eye Disease Investigator Group. Randomized trial of treatment of amblyopia in children age 7–17 years. Arch Ophthalmol 2005; 123: 437–447.
Pediatric Eye Disease Investigator Group. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol 2003; 121: 603–611.
Pediatric Eye Disease Investigator Group. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology 2003; 110(11): 2075–2087.
Arikan G, Yaman A, Berk AT . Efficacy of occlusion treatment in amblyopia and clinical risk factors affecting the results of treatment. Strabismus 2005; 13(2): 63–69.
Simmers AJ, Gray LS, McGraw PV, Winn B . Functional visual loss in amblyopia and the effect of occlusion therapy. Invest Ophthalmol Vis Sci 1999; 40(12): 2859–2871.
Dorey SE, Adams GG, Lee JP, Sloper JJ . Intensive occlusion therapy for amblyopia. Br J Ophthalmol 2001; 85(3): 310–313.
Harwerth RS, Fredenburg PM . Binocular vision with primary microstrabismus. Invest Ophthalmol Vis Sci 2003; 44(10): 4293–4306.
Grossberg S . Why do parallel cortical systems exist for the perception of static form and moving form? Percept Psychophs 1991; 49(2): 117–141.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cleary, M., Moody, A., Buchanan, A. et al. Assessment of a computer-based treatment for older amblyopes: the Glasgow Pilot Study. Eye 23, 124–131 (2009). https://doi.org/10.1038/sj.eye.6702977
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702977
Keywords
This article is cited by
-
Rehabilitation of visual functions in adult amblyopic patients with a virtual reality videogame: a case series
Virtual Reality (2023)
-
Efficacy of vision therapy for unilateral refractive amblyopia in children aged 7–10 years
BMC Ophthalmology (2022)
-
Study protocol for a randomized controlled trial of the NEIVATECH virtual reality system to improve visual function in children with anisometropic amblyopia
BMC Ophthalmology (2022)
-
Portable rotating grating stimulation for anisometropic amblyopia with 6 months training
Scientific Reports (2021)
-
Clinical investigation plan for the use of interactive binocular treatment (I-BiT) for the management of anisometropic, strabismic and mixed amblyopia in children aged 3.5–12 years: a randomised controlled trial
Trials (2019)