Abstract
Aim
To compare the ability of scanning laser polarimeter (SLP) with variable corneal compensation (GDx VCC) and optical coherence tomograph (Stratus OCT) to discriminate between eyes with band atrophy (BA) of the optic nerve and healthy eyes.
Methods
The study included 37 eyes with BA and temporal visual field (VF) defects from chiasmal compression, and 29 normal eyes. Subjects underwent standard automated perimetry (SAP) and retinal nerve fibre layer (RNFL) scans using GDx VCC and Stratus OCT. The severity of the VF defects was evaluated by the temporal mean defect (TMD), calculated as the average of 22 values of the temporal total deviation plot on SAP. Receiver operating characteristic (ROC) curves were calculated. Pearson's correlation coefficients were used to evaluate the relationship between RNFL thickness parameters and the TMD.
Results
No significant difference was found between the ROC curves areas (AUCs) for the GDx VCC and Stratus OCT with regard to average RNFL thickness (0.98 and 0.99, respectively) and the superior (0.94; 0.95), inferior (0.96; 0.97), and nasal (0.92; 0.96) quadrants. However, the AUC in the temporal quadrant (0.77) was significantly smaller (P<0.001) with GDx VCC than with Stratus OCT (0.98). Lower TMD values were associated with smaller RNFL thickness in most parameters from both equipments.
Conclusion
Adding VCC resulted in improved performance in SLP when evaluating eyes with BA, and both technologies are sensitive in detecting average, superior, inferior, and nasal quadrant RNFL loss. However, GDx VCC still poorly discriminates RNFL loss in the temporal quadrant when compared with Stratus OCT.
Similar content being viewed by others
Introduction
Changes in the structural appearance of the optic nerve head and retinal nerve fibre layer (RNFL) often accompany the development of visual field (VF) loss in patients with chiasmal compression indicating permanent damage of the anterior visual pathways. Patients with extensive mid-chiasmal lesions affecting crossed nerve fibres, and showing bitemporal hemianopia, but with preserved nasal field, generally demonstrate very distinctive changes in the peripapillary area. In these cases, the crossed nerve fibres are lost with preservation of the uncrossed nerve fibres, which originate in the temporal hemiretina and penetrate the optic nerve through the superior and inferior arcuate fibre bundles. Thus, RNFL loss occurs predominantly in the nasal and temporal side of the optic disc. The pattern is identified on ophthalmoscopy as band atrophy (BA) of the RNFL,1 and is important in the diagnosis of chiasmal compression and in estimating the chances of improvement of VF defects after decompression of the optic pathway. This characteristic pattern of RNFL loss may also serve as a model for evaluating the ability of any instrument to measure RNFL thickness accurately, particularly in the nasal and temporal quadrants of the optic nerve.
We demonstrated recently that scanning laser polarimetry (SLP) failed to detect properly the characteristic pattern of BA of the optic nerve,2 whereas the first commercial version of optical coherence tomography (OCT) performed better in the identification of such pattern.3 However, both these technologies have recently been endowed with significant hardware and software improvements: the introduction of variable corneal compensation (VCC) has improved GDx (Laser Diagnostic Technologies Inc., San Diego, CA, USA) diagnostic accuracy compared with the earlier version of this instrument. Likewise, the new Stratus OCT (Carl Zeiss Meditec, Inc, Dublin, CA, USA) features a number of improvements, such as higher resolution, shorter acquisition time, and an increased number A scans.4
The purpose of this study was to evaluate the ability of GDx VCC and Stratus OCT to differentiate eyes with BA of the optic nerve from normal eyes. We also evaluated the relationship between RNFL thickness and the severity of VF damage in patients with BA. Finally, the ability of GDx VCC RNFL thickness parameters to detect patients with BA was compared with that of Stratus OCT RNFL thickness parameters using the same sample of subjects.
Patients and methods
This was an observational prospective cross-sectional study. Participants were recruited for examination at the Department of Ophthalmology of the University of São Paulo Medical School between May 2003 and October 2005. Approval from the Institutional Review Board Ethics Committee was obtained for the study. The study followed the principles of the Declaration of Helsinki and informed consent was obtained from all participants.
A total of 37 eyes from 37 patients (21 men) with temporal hemianopia from chiasmal compression and 29 eyes from 29 normal (16 men) age- and sex-matched controls were studied. All patients with history of chiasmal lesions had already been submitted to previous treatment of the suprasellar lesion and had stable VF defects and visual acuity (VA) for at least 1 year before study entry.
All subjects underwent a complete ophthalmologic examination including VF evaluation. VF testing was performed using the Goldmann perimeter (GP) (Haag-Streit AG, Bern, Switzerland). The V-4–e, I-4–e, I-3–e, I-2–e, and I-1–e stimuli were used to draw the isopters. Kinetic determinations were followed by static presentation of the stimuli particularly in the central 30° area, in order to search for localized defects. All patients were also submitted to standard automated perimetry (SAP) using the 24–2 full-threshold strategy (Humphrey Field Analyzer, Carl-Zeiss Meditec, Dublin, CA, USA). VF and RNFL examinations were performed on the same day or within a maximum period of 2 weeks.
Inclusion criteria for the study included best-corrected VA of 20/30 or better in the study eye; age between 18 and 72 years, spherical refraction within ±5D, cylinder refraction within ±4D, intraocular pressure less than 22 mmHg, and reliable VF. A reliable Humphrey VF test was defined as one with fewer than 25% fixation losses, false-positive responses, or false-negative responses. Patients with previous history of intraocular pressure elevation, with clinical signs of glaucomatous optic neuropathy or optic disc anomaly were excluded.
Patients with BA were required to have complete or partial temporal hemianopia on Humphrey and GP and a nasal hemifield within normal limits on both tests. A normal nasal hemifield on GP was defined by the presence of normal I-4–e, I-2–e, and I-1–e isopters. On SAP, a normal hemifield was defined as the absence of any cluster of at least three points with P<5% on the pattern deviation plot. Only one eye of each patient was selected for analysis. In 31 patients, only one eye met the inclusion criteria. For the six patients in whom both eyes fulfilled the inclusion criteria, one eye was randomly selected for analysis. The severity of VF defect in patients with BA was evaluated by calculating the temporal mean defect (TMD). This was performed by averaging the values of the total deviation plot for the 22 temporal points of the SAP 24–2 test, excluding the two points immediately above and below the blind spot.
The control group consisted of normal healthy volunteers recruited from among the hospital staff. All normal subjects had normal ophthalmic examination and normal SAP VFs. A normal SAP VF was defined as a pattern standard deviation within the 95% confidence limits and a glaucoma hemifield test result within normal limits. Healthy control eyes also had a healthy appearance of optic disc and RNFL. One eye of each healthy subject was included for analysis and the selection between right or left eye was performed to match the selection in patients with BA.
OCT scanning
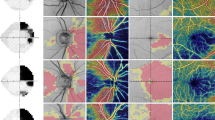
Subjects underwent ocular imaging with dilated pupils using a commercially available Stratus OCT. OCT employs the principles of low-coherence interferometry and is analogous to ultrasound B-mode imaging, but uses light instead of sound to acquire images of ocular structures. The basic principles and technical characteristics have been described previously.4 Quality assessment of Stratus OCT scans was evaluated by an experienced examiner. Good-quality scans had to have focused images and signal strength equal to or higher than seven, and presence of a centred circular ring around the optic disc for RNFL scans.
The fast RNFL algorithm was used to obtain RNFL thickness measurements with Stratus OCT. Three images were acquired from each subject, with each image consisting of 256 A-scans along a 3.4-mm-diameter circular ring around the optic disc. Peripapillary RNFL thickness parameters automatically calculated by existing Stratus OCT software (version 4.0.1) and evaluated in this study were average thickness (360° measure), temporal quadrant thickness (316–45°), superior quadrant thickness (46–135°), nasal quadrant thickness (136–225°), inferior quadrant thickness (226–315°), and thickness for each of 12 O'clock-hour positions with the 3 o'clock position as nasal, 6 o'clock position as inferior, 9 o'clock position as temporal, and 12 o'clock position as superior.
SLP
The thickness of the peripapillary RNFL was also determined using a commercially available GDx VCC with software version 5.4.0, which uses variable corneal compensation. The spherical-equivalent refractive error of each eye was entered into the software to allow the GDx VCC to focus on the retina. General principles of SLP and its operation have been reported previously.5 In brief, the device uses a diode laser with a wavelength of 780 nm to create the polarized laser beam,6 which is directed to the retina, and the reflected light that double-passes the RNFL is detected to obtain the retardation image at that point. The GDx VCC incorporates the method of VCC, which has been described elsewhere.7 To measure the eye-specific corneal polarization axis and magnitude, images are first acquired from the macula, without any compensation. The resulting retardation profile represents the additive effect of cornea, lens, and Henle fibre birefringence. Retardation values (nm) are converted into thickness values (μm). A mean image was created from three images obtained from each subject. The quality of the reflectance image and retardance image for the cornea and macula was reviewed for even illumination, centring, and appropriate corneal compensation. The thickness data are transferred into a colour-coded image. Thin areas of the RNFL relate to darker colours (blue–black), and thicker areas to bright colours (yellow–orange). The operator has to position a blue ellipse around the margin of the optic disc. A second green ellipse with a given magnification factor of 1.75 disc diameter is displayed automatically peripherally to the blue centre ellipse.
The thickness image is divided into four segments: superior 120°(for 0°at a 12 O'clock position: 295–55°), nasal 70°(55–125°), inferior 120°(125–245°), and temporal 50°(245–295°). These segments are used to calculate the different parameters of the nerve fibre analysis. The GDx VCC parameters investigated in this study were, temporal, superior, nasal, inferior, temporal (TSNIT) average, superior average, temporal average, inferior average, nasal average, and nerve fibre indicator (NFI). The NFI is calculated using a support vector machine algorithm based on several RNFL measures and assigns a number from 0 to 1000 to each eye. The higher the NFI, the greater the likelihood that the patient has an abnormality in the RNFL. The studied eye of each patient and each normal control was examined six times. The pupils were undilated. The quality of all images as determined by the GDx quality check was consistently 90% or higher. The best three images, where the optic disc was centred in such a way that it could be easily aligned, were chosen to create a mean image. All images were taken by the same operator that also positioned the ellipse around the margin of the optic disc.
Statistical analysis
Descriptive statistics included mean±SD for the variables that were normally distributed. Analysis of histograms and the Shapiro–Wilk test were used to evaluate the normality assumption.
RNFL thickness values of eyes with BA and normal controls were compared using Student's t-tests. Receiver operating characteristic (ROC) curves were used to describe the ability of Stratus OCT and GDx VCC parameters to discriminate BA from healthy eyes. The method of DeLong et al8 was used to compare areas under the ROC curves (AUCs). For each parameter, sensitivities at fixed specificities of 80 and 95% were calculated. Pearson's correlation coefficients were used to evaluate the relationship between RNFL thickness parameters and severity of VF loss as measured by the TMD.
A P-value less than 0.05 was considered as statistically significant. Results of statistical significance were provided after Bonferroni's correction based on the number of comparisons within each analysis. Statistical analyses were performed using software SPSS v.14.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 37 eyes with temporal hemianopia and 29 control eyes were studied. Thirty-two patients had pituitary adenoma, three had craniopharyngioma, and two had suprasellar menigioma. The SD age was 44.4±11.7 years (range 21–72) in BA patients and 39.6±10.9 years (range 20–69) in normal subjects (P=0.1; Student's t-test). On GP, six eyes had complete temporal hemianopia; three eyes had almost complete hemianopia with only a small inferior temporal reminiscent of field with V/4e target; six eyes had a temporal reminiscent of field with V/4e and I/4e targets (complete defect up to the I/3e stimulus), and the others had milder defects in the upper temporal field involving I/3e, I/2e, and I/1e targets. On SAP, 15 eyes had complete temporal hemianopia, six had a defect of approximately one quadrant, and 16 eyes had a defect involving less than one quadrant of VF. Mean SD of SAP mean deviation and SAP TMD were −8.25±5.13 dB and −18.06±10.67 dB, respectively. Fundoscopic examination revealed signs of BA of the optic disc and RNFL in all 37 eyes with temporal hemianopic field defect.
Table 1 shows RNFL thickness measurements in eyes with BA and healthy eyes using GDx VCC. After Bonferroni's correction (α=0.008; six comparisons), all RNFL thickness parameters were significantly lower for eyes with BA than for normal eyes. AUCs and sensitivities at fixed specificities are also shown in Table 1. The AUCs for the GDx VCC parameters TSNIT, NFI, superior average, inferior average, and nasal average measured, 0.98, 0.96, 0.94, 0.96, and 0.92 respectively. There were no statistically significant differences between the AUCs for these parameters (P>0.05 for all comparisons), but the AUC of the temporal quadrant was significantly smaller (0.76) than the AUCs of the other GDx parameters (P<0.05, for all comparisons).
Table 2 shows RNFL thickness measurements in eyes with BA and healthy eyes using Stratus OCT. After Bonferroni's correction (α=0.003; 17 comparisons), all RNFL thickness parameters were significantly lower for eyes with BA than for normal eyes. AUCs and sensitivities at fixed specificities are also shown in Table 2. The AUCs for the Stratus OCT RNFL parameters average thickness superior thickness, temporal thickness, inferior thickness, and nasal thickness measured 0.99, 0.95, 0.98, 0.96, and 0.98, respectively. There were no statistically significant differences between the AUCs for these parameters (P>0.05 for all comparisons).
No statistically significant difference (P=0.47) was observed between the GDx VCC parameter with the largest AUC (TSNIT, 0.98) and the Stratus OCT parameter with the largest AUC (average thickness, 0.99). Likewise, a comparison of the AUCs for each quadrant revealed no significant differences with regard to the superior, inferior, and nasal quadrant measurements with either technology (P>0.05 for all comparisons), but the AUC of the temporal quadrant was significantly smaller with GDx VCC (0.76) than with Stratus OCT (0.98) (P<0.001).
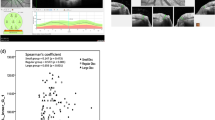
Table 4 shows the associations between RNFL thickness parameters and TMD values using both GDx VCC and Stratus OCT. For GDx VCC, the parameter superior average thickness showed the highest correlation with severity of VF loss (r=0.716, R2=51%, P<0.001). Figure 1 shows a scatterplot of superior average vs TMD values. For Stratus OCT, the highest correlation was observed for the average thickness parameter (r=0.629, R2=40%, P<0.001), with lower values of thickness associated with lower TMD values. Figure 2 shows a scatterplot of Stratus OCT average thickness parameter measurements vs TMD values.
Discussion
In the present study, we demonstrate that both GDx VCC and Stratus OCT RNFL thickness measurements were significantly lower for eyes with BA of the optic nerve than for healthy control eyes with regard to all parameters. The most efficient RNFL thickness parameters in the detection of damage in eyes with BA were average TSNIT, NFI, and superior average (GDx VCC) and total average, nasal, and temporal quadrant thickness (Stratus OCT). To our knowledge, this is the first study to provide a comparison of the diagnostic accuracy of the two instruments, GDx VCC and Stratus OCT in eyes with BA of the optic nerve, applied to the same population. Previous studies using the SLP with fixed corneal compensation (FCC) and OCT in such eyes indicated a superior ability of OCT when compared with GDx. In a series of 19 eyes with severe BA, we demonstrated that GDx with FCC failed to detect RNFL loss in the temporal quadrant 2. RNFL thickness in the temporal quadrant was not significantly different from that of normal eyes, despite the fact that all eyes had severe RNFL loss in that segment on ophthalmoscopy. In another study, with a similar series of eyes, an earlier version of OCT (OCT 1) performed much better at discriminating this pattern of RNFL loss with a significant difference in the average measurements and in each of the four quadrants of the optic nerve.3
The current study shows GDx VCC to be superior to GDx with FCC, considering that a significant difference between eyes with BA and normal eyes was observed for all parameters (Table 1). On the other hand, our findings confirmed Stratus OCT's previously reported efficacy at discriminating between eyes with BA and normal eyes with a highly significant difference (P<0.001) in all parameters.3, 9
Medeiros et al10 compared the ability of GDx VCC, the confocal laser ophthalmoscope and Stratus OCT to discriminate between healthy eyes and eyes with glaucomatous VF loss. The AUCs and the sensitivities at high specificities were similar for the best parameters from each instrument. In a comparison of GDx with FCC and GDx VCC, Weinreb et al11 found the latter to discriminate glaucomatous from normal eyes more efficiently, but found no significant difference in temporal average measurements and only a modest difference in nasal average measurements (P=0.02) between normal and glaucomatous eyes.
Patients with glaucoma are not ideal subjects for studies on equipment designed to evaluate the temporal RNFL thickness as this region is not preferentially affected in glaucoma. Leal et al12 compared the AUCs of RNFL parameters using GDx with FCC, OCT 1, and Stratus OCT in 23 eyes with severe BA of the optic nerve and found that the AUC for the RNFL average measurements was significantly larger for Stratus OCT (0.99) than for OCT 1 (0.95) or GDx with FCC (0.88). The AUCs for the superior, temporal, inferior, and nasal quadrant were 0.98, 0.99, 0.99, and 0.98, respectively, for Stratus OCT; 0.91, 0.73, 0.97, and 0.91 for OCT 1 and 0.87, 0.52, 0.89, and 0.71 for GDx with FCC. In other words, the performance of GDx was significantly inferior to that of either OCT 1 or Stratus OCT.
Our study shows that although the mean values of all GDx VCC parameters were significantly different for eyes with BA and normal eyes, a comparison of AUCs shows Stratus OCT to be markedly superior to GDx VCC when measuring the temporal quadrant of the optic nerve (Table 3). With regard to average RNFL thickness and the superior, inferior, and nasal quadrants, the two technologies displayed comparable diagnostic accuracy, but Stratus OCT performed significantly better when measuring the temporal quadrant: AUCs in the temporal quadrant measured 0.98 with Stratus OCT and 0.76 with GDx VCC, respectively (P<0.001). The reason for the low sensitivity of GDx VCC in that region is not fully understood, but some workers have reported that VCC measurements can display an atypical retardation pattern,13, 14 which it may be hypothesized, would tend to interfere with the relatively thinner temporal area.
The evaluation of eyes with BA provides a potential model for investigating the ability of imaging instruments to assess accurately eyes with RNFL damage, particularly in the temporal and nasal areas of the optic disc. The ability to assess RNFL loss in these areas is important for the study of neuro-ophthalmological conditions with a predilection for RNFL damage in these sectors, such as chiasmal and optic tract compression, heredodegenerative diseases, and toxic, nutritional, compressive, and even inflammatory optic neuropathies. The patients included in our study had already been submitted to chiasmal decompression and retained severe and long-standing hemianopic VF defects, with corresponding structural damage observed on ophthalmoscopy as BA of the optic nerve. Therefore, VF defects in our cases were likely to be permanent. Significant correlations were found between GDx VCC and Stratus OCT RNFL thickness measurements and the severity of VF loss in patients with BA of the optic nerve (Table 4). These correlations were generally similar to previously reported structure–function relationships between Stratus OCT parameters and severity of VF loss in patients with glaucoma. Schlottmann et al15 reported an R2 of 0.49 and 0.47 for the associations between global and sectoral GDx VCC RNFL thickness, respectively, and VF mean defect in a group of 51 glaucomatous patients. In our study, the GDx VCC parameter superior average thickness had the best association with the degree of field loss (R2=0.51). For the Stratus OCT RNFL thickness parameters, the best association was observed for the parameter average thickness (R2=0.40). The significant relationships found between GDx VCC and Stratus OCT parameters and measures of visual function in eyes with BA suggest that these instruments could potentially be used to evaluate severity of disease in these patients. Future studies should be directed at studying the potential use of these measurements to monitor patients with BA.
In conclusion, both GDx VCC and Stratus OCT RNFL thickness measurements were able to differentiate eyes with BA from normal controls. Although the addition of VCC has improved GDx discrimination ability, the technology is still less efficient than Stratus OCT when it comes to measuring the temporal quadrant. Although GDx VCC seems reliable when evaluating the overall RNFL loss in patients with BA of the optic nerve, clinicians should still prefer Stratus OCT measurements when the purpose is to evaluate RNFL in the temporal area of the optic nerve head.
References
Unsold R, Hoyt WF . Band atrophy of the optic nerve. The histology of temporal hemianopsia. Arch Ophthalmol 1980; 98: 1637–1638.
Monteiro ML, Medeiros FA, Ostroscki MR . Quantitative analysis of axonal loss in band atrophy of the optic nerve using scanning laser polarimetry. Br J Ophthalmol 2003; 87: 32–37.
Monteiro ML, Leal BC, Rosa AA, Bronstein MD . Optical coherence tomography analysis of axonal loss in band atrophy of the optic nerve. Br J Ophthalmol 2004; 88: 896–899.
Jaffe GJ, Caprioli J . Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol 2004; 137: 156–169.
Weinreb RN, Shakiba S, Zangwill L . Scanning laser polarimetry to measure the nerve fiber layer of normal and glaucomatous eyes. Am J Ophthalmol 1995; 119: 627–636.
Weinreb RN, Dreher AW, Coleman A, Quigley H, Shaw B, Reiter K et al. Histopathologic validation of Fourier-ellipsometry measurements of retinal nerve fiber layer thickness. Arch Ophthalmol 1990; 108: 557–560.
Zhou Q, Weinreb RN . Individualized compensation of anterior segment birefringence during scanning laser polarimetry. Invest Ophthalmol Vis Sci 2002; 43: 2221–2228.
DeLong ER, DeLong DM, Clarke-Pearson DL . Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845.
Monteiro ML, Leal BC, Moura FC, Vessani RM, Medeiros FA . Comparison of retinal nerve fibre layer measurements using optical coherence tomography versions 1 and 3 in eyes with band atrophy of the optic nerve and normal controls. Eye 2007; 21: 16–22.
Medeiros FA, Zangwill LM, Bowd C, Weinreb RN . Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and stratus OCT optical coherence tomograph for the detection of glaucoma. Arch Ophthalmol 2004; 122: 827–837.
Weinreb RN, Bowd C, Zangwill LM . Glaucoma detection using scanning laser polarimetry with variable corneal polarization compensation. Arch Ophthalmol 2003; 121: 218–224.
Leal BC, Moura FC, Monteiro ML . Comparison of scanning laser polarimetry, optical coherence tomography 1 and Stratus OCT for the detection of axonal loss in band atrophy of the optic nerve. Arq Bras Oftalmol 2006; 69 (4): 531–537.
Bagga H, Greenfield DS, Feuer WJ . Quantitative assessment of atypical birefringence images using scanning laser polarimetry with variable corneal compensation. Am J Ophthalmol 2005; 139: 437–446.
Toth M, Hollo G . Enhanced corneal compensation for scanning laser polarimetry on eyes with atypical polarisation pattern. Br J Ophthalmol 2005; 89: 1139–1142.
Schlottmann PG, De Cilla S, Greenfield DS et al. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by scanning laser polarimetry. Invest Ophthalmol Vis Sci 2004; 45: 1823–1829.
Acknowledgements
This work was supported by a grant from Fundação de Amparo a Pesquisa do Estado de São Paulo FAPESP (no. 05/55326-1), São Paulo, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monteiro, M., Moura, F. Comparison of the GDx VCC scanning laser polarimeter and the stratus optical coherence tomograph in the detection of band atrophy of the optic nerve. Eye 22, 641–648 (2008). https://doi.org/10.1038/sj.eye.6702694
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702694
Keywords
This article is cited by
-
Optical coherence tomography impacts the evaluation of visual pathway tumors
Neurosurgical Review (2018)
-
Correlation between multifocal pattern electroretinography and Fourier-domain OCT in eyes with temporal hemianopia from chiasmal compression
Graefe's Archive for Clinical and Experimental Ophthalmology (2013)
-
The optic nerve head in acquired optic neuropathies
Nature Reviews Neurology (2010)