Abstract
Purpose:
To evaluate the effects of topical latanoprost, travoprost, and bimatoprost on the blood–aqueous barrier and central corneal thickness (CCT) of patients with primary open-angle glaucoma (POAG) and ocular hypertension (OHT).
Design:
Prospective, randomized, masked-observer, crossover clinical trial.
Methods:
A total of 34 phakic patients with POAG or OHT with no previous history of intraocular surgery or uveitis completed the study. Patients were randomized to use latanoprost 0.005%, travoprost 0.004%, or bimatoprost 0.03% once daily (2000 hours) for 1 month, followed by a washout period of 4 weeks between each drug. Aqueous flare was measured with a laser flare metre. CCT was calculated as the average of five measurements using ultrasound pachymetry. All measurements were performed by a masked observer (1000 h).
Results:
There were no statistically significant differences between baseline mean IOP, mean CCT, and mean flare values among the groups. There was no statistically significant increase in mean flare values from baseline in all groups (P>0.05). There were no statistically significant differences between mean flare values among the groups (P>0.05). All medications significantly reduced the mean IOP from baseline (P<0.0001). IOP reduction obtained with travoprost (7.3±3.8 mmHg) was significantly higher than that obtained with latanoprost (4.7±4.2 mmHg) (P=0.01). A statistically significant reduction in mean CCT (0.6±1.3%) from baseline was observed when patients instilled bimatoprost (P=0.01).
Conclusions:
Latanoprost, travoprost, and bimatoprost had no statistically significant effect on the blood–aqueous barrier of phakic patients with POAG or OHT. Bimatoprost may be associated with a clinically irrelevant reduction in mean CCT.
Similar content being viewed by others
Introduction
Prostaglandin (PG) analogues have become widely used in the treatment of primary open-angle glaucoma (POAG) and ocular hypertension (OHT) owing to a robust IOP-lowering effect and few systemic side effects. 1, 2, 3, 4 However, local adverse effects have been reported with PG analogues, including conjunctival hyperemia, iris hyperpigmentation, and eyelash growth.1, 5, 6, 7 Among the serious PG-induced side effects are the disruption of the blood–aqueous barrier (BAB) and the development of cystoid macular oedema (CME), which have been described in susceptible eyes, especially in aphakic or pseudophakic patients.8, 9, 10, 11, 12, 13, 14, 15, 16, 17 However, there is controversy regarding the effects of PG analogues on the BAB of phakic patients with no previous history of surgery or uveitis.18, 19, 20, 21, 22, 23, 24 Furthermore, it has been reported that topical application of PG analogues may also reduce central corneal thickness (CCT),25 which could influence IOP measurements by applanation tonometry.26, 27, 28
While it is important to evaluate the ocular hypotensive efficacy of PG analogues, it is also necessary to determine their effects on the ocular surface and intraocular inflammatory reaction of phakic patients. The aim of this study was to evaluate the effects of PG analogues on the BAB and CCT of phakic patients with POAG or OHT.
Methods
This prospective, randomized, masked-observer, crossover clinical trial was conducted at the Glaucoma Service of the University of Campinas. The study was performed in accordance with the Declaration of Helsinki Principles after receiving approval from the Ethics Committee of the University of Campinas. Written informed consent was obtained from each patient before inclusion in the study.
The present study included 40 patients with POAG or OHT and age greater than 18 years. Glaucoma was defined as an untreated IOP of more than 21 mmHg in at least one eye measured on two consecutive occasions separated by an interval of at least 2 h but no more than 4 weeks, glaucomatous changes in the visual field or optic disc, or defects in the retinal nerve fibre layer. OHT was defined as an untreated IOP of more than 21 mmHg with normal visual field, optic disc, and retinal nerve fibre layer examinations.
Exclusion criteria included best-corrected visual acuity worse than 20/80 in either eye; corneal abnormalities; uncontrolled systemic disease; active ocular diseases other than POAG or OHT; contact lens use; pregnancy, planning a pregnancy, breastfeeding, or lack of usage of an adequate birth control method in women; functionally significant visual field loss; history of ocular trauma or uveitis; previous ocular surgery or laser treatment; and concomitant use of ocular medications.
Before study entry, all patients underwent a complete ophthalmologic examination including slitlamp biomicroscopy, applanation tonometry, fundus examination, and automated visual field examination (Humphrey 750, programme 24-2, SITA strategy). Patients with IOP greater than 30 mmHg in either eye during the eligibility phase were excluded on the basis of potential risk during the study.
If patients were eligible but were using antiglaucoma medications, hypotensive therapy was discontinued. Required washout periods before the baseline visit were 4 weeks for β-adrenergic antagonists and PG analogues, 2 weeks for adrenergic agonists, and 5 days for cholinergic agonists and carbonic anhydrase inhibitors. A safety check with IOP measurement was required after 2 weeks for all patients undergoing a 4-week washout. At that time, patients whose IOPs had risen to levels deemed to be detrimental were excluded from the study. No other IOP-reducing therapy was permitted during the study. If both eyes of a patient were eligible for inclusion in the study, the same medication was prescribed for both eyes, although only one eye per patient (chosen at random) was included in the analysis.
Using a list of random numbers, patients were randomized to receive one of the following treatment sequences: (1) A, B, C; (2) A, C, B; (3) B, A, C; (4) B, C, A; (5) C, A, B; or (6) C, B, A; where A= latanoprost 0.005% (Xalatan®; Pfizer Inc., New York, NY, USA), B=bimatoprost 0.03% (Lumigan®; Allergan Inc., Irvine, CA, USA), and C=travoprost 0.004% (Travatan®; Alcon Inc., Ft Worth, TX, USA). Study medications were packaged in commercially available, labelled containers. Before dispensing, latanoprost was stored refrigerated at 2–8°C, and patients were instructed to conserve it under refrigeration. Bimatoprost and travoprost were stored at room temperature. To preserve masking, a designated unmasked coordinator (ESA)—who did not perform any study evaluation or assessment—received randomization codes, dispensed the medications, and instructed the patients on how to use and store the containers. Participants were instructed to instill the eye drops once daily (2000 hours).
Each trial drug was administered for 4 weeks. At the end of the fourth week, measurements of IOP, flare, and CCT were recorded. Patients were washed out for 4 weeks between each regimen of medications. At each visit, all patients underwent the following sequence of exams: Snellen visual acuity measurement, slit lamp biomicroscopy, flare, IOP, and CCT measurements. The measurements were performed at the same time (1000 hours) at all visits by a masked observer (PTPPF).
The IOP was measured using a Goldmann applanation tonometer. A laser flare metre (FM 500; Kowa Co Ltd, Tokyo, Japan) was used to determine the status of the BAB at all follow-up visits. The flare measurements were repeated seven times, the highest and lowest values were excluded, and the mean of the five remaining values was adopted as a ‘flare value’ for statistical analysis. Five consecutive ultrasound pachymetry (Pachette DGH 500, DGH Technology Inc., Philadelphia, PA, USA) measurements of CCT were obtained from each eye, and a mean value was then calculated.
Before the study, it was determined that a sample size of 14 patients had 90% power to detect a 20% difference in aqueous flare measurements between the groups at a significance level of α=0.05, using the estimated variability determined in a previous study.29 Statistical analysis was performed with the paired t test. P<0.05 was considered statistically significant. Data are presented as mean±SD, unless stated otherwise.
Results
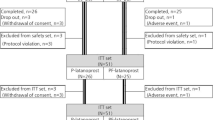
We enrolled 40 patients in this crossover comparison. Six patients were excluded from the study: four of them had IOPs >30 mmHg at baseline, and two of them moved and were not able to return for follow-up visits. Among the 34 patients who completed the trial, 17 (50%) had OHT, and 17 (50%) had POAG. Twenty were female (58.8%) and 14 male (41.2%); 21 were white (61.8%) and 13 black (38.2%) patients. The average age was 57.8±9.6 years (range=39–76 years). Washout of antiglaucoma medications was required for 17 patients (50%): 10 were on timolol, three on travoprost, two on latanoprost, one on bimatoprost, one on the fixed combination of timolol and dorzolamide, and one on the fixed combination of timolol and brimonidine.
Table 1 lists the mean IOP at baseline and 4 weeks post-treatment for each group. There was no statistically significant difference between mean baseline IOPs for each group (P=0.484). At the end of the fourth week of treatment, all three PG analogues showed significant IOP reductions compared with baseline (P<0.00001). Travoprost resulted in a significantly greater mean IOP reduction (7.3±3.8 mmHg) than latanoprost (4.7±4.2 mmHg) (P=0.014). Mean IOP reductions did not differ between latanoprost and bimatoprost (6.4±4.1 mmHg) (P=0.153), or bimatoprost and travoprost (P=0.287).
Table 2 lists the mean amount of aqueous flare at baseline and 4 weeks post-treatment for each group. There were no significant differences in mean flare values between baseline and follow-up visits for the bimatoprost, latanoprost, and travoprost groups (P>0.050). Moreover, there were no significant differences in mean flare measurements between eyes receiving bimatoprost, latanoprost, and travoprost (P>0.069). Aqueous flare values greater than 26 p/ms (indicative of uveitis, according to the manufacturer) were not observed with any drug.
Changes in corneal thickness throughout the study are displayed in Table 3. There was a statistically significant decrease (0.6±1.3%) in mean CCT with bimatoprost 4 weeks after treatment (P=0.010). Latanoprost (P=0.109) and travoprost (P=0.354) did not induce significant changes in mean CCT from baseline.
Discussion
The onset of intraocular inflammation induced by PG analogues is a frequently debated event that is related to the safety of these drugs. Prostaglandins are chemical mediators physiologically involved in the inflammatory process.11 It has been shown that PG analogues can cause intraocular inflammation with rupture of the blood–aqueous and blood–retinal barriers in predisposed eyes.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 21
The mechanisms associated with PG-induced intraocular inflammation have not been completely elucidated. It has been suggested that PGF2α stimulates the release of PGE2, which in turn stimulates the release of arachidonic acid by activating phospholipase II.30 Arachidonic acid may promote the increase of eicosanoids as well as other proinflammatory mediators in the eye, ultimately leading to changes in the blood–aqueous and blood–retinal barriers.30
Clinically observable flare in the anterior chamber has been seen in some the patients treated with latanoprost following anterior segment surgery.17 Recently, we reported a significant increase in flare values measured with the laser flare metre in pseudophakic and aphakic patients receiving bimatoprost, latanoprost, and travoprost for 6 months.8 The disruption of the BAB was significantly associated with the development of CME, which resolved after the administration of nonsteroidal anti-inflammatory drugs.8
Although changes in the BAB and BRB have been documented in pseudophakic and aphakic eyes using PG analogues, there is still some controversy regarding the effects of PG analogues on phakic patients without a previous history of a BAB break. Several studies employing different methodology have failed to detect any significant effect of PGF2α and latanoprost on the BAB of phakic patients.22, 23 Linden et al24 evaluated the effect of 6–12 months treatment with latanoprost on the BAB of 16 patients with OHT or glaucoma and did not report changes in aqueous flare measured by the laser flare metre. Widengard et al22 investigated the effects of latanoprost and dipivefrin on the aqueous flare of 22 patients with POAG or OHT. Patients were allocated to either once daily evening administration of latanoprost monotherapy (50 μg/ml) or twice daily dipivefrin monotherapy (1 mg/ml) for 2 weeks, followed by 2 weeks of concomitant use of both drugs. No change in aqueous flare was observed with either drug, alone, or in combination.
On the other hand, Cellini et al20 evaluated the BAB of glaucomatous eyes after treatment with PG analogues using the laser flare metre. In total, 60 patients with chronic open-angle glaucoma were randomly assigned to latanoprost 0.005% (n=20), travoprost 0.004% (n=20), and bimatoprost 0.03% (n=20). The authors observed that 6 months of treatment with latanoprost, travoprost, and bimatoprost significantly increased the aqueous flare from baseline in phakic patients with chronic open-angle glaucoma. However, the authors do not mention if patients with a previous history of intraocular surgery/laser were allowed to enter the study. The inclusion of patients with a previous history of BAB breakdown might justify the increased mean flare values observed by Cellini et al20 following the use of PG analogues.
In our series, no statistically significant increase in mean flare values from baseline was found when patients used bimatoprost, latanoprost, or travoprost for 4 weeks. None of the 34 included patients had a previous history of BAB breakdown. Furthermore, there were no statistically significant differences between mean flare values in bimatoprost-, latanoprost-, and travoprost-treated eyes.
It is possible that the reason for the discrepancy between these and Cellini's findings rests on the length of follow-up: while ours was a short-term study with 4 weeks of follow-up, their patients were observed for 6 months. In fact, the occurrence of clinically detectable uveitis or CME in phakic patients without a previous history of BAB breakdown is rare. Smith et al19 retrospectively investigated 505 glaucoma patients with no prior uveitis and observed that five (1.0%) developed uveitis after the use of latanoprost, compared with three of 13 (23.1%) patients with prior uveitis but inactive at the time of the study. In both groups, the uveitis tended to occur a mean of 3 months following the use of latanoprost, indicating that BAB changes might have been found in our series if the follow-up was longer.
This series also demonstrated that bimatoprost, latanoprost, and travoprost significantly reduced the IOP of phakic patients with POAG or OHT after 1 month of treatment. The mean IOP reduction was significantly higher for travoprost compared with latanoprost after 4 weeks of treatment, but was statistically equivalent to bimatoprost. When latanoprost and bimatoprost were compared, there were no statistically significant differences in mean IOP reduction. The mean IOP reduction obtained with latanoprost (21.3%) was lower than the one reported in the literature (26–33%),31 suggesting that this study may have included a higher rate of nonresponders to this drug.
Viestenz et al25 investigated the influence of PGF2α analogues on the CCT in a nonrandomized, controlled, cross-sectional study with 403 eyes from 208 consecutive patients, and suggested that the topical application of these substances significantly reduced CCT. In a double-masked trial, subjects with POAG or OH were randomly assigned to treatment with latanoprost (n=127), fixed-combination of latanoprost and timolol (n=116), or timolol (n=126) for 1 year.32 This study reported changes in CCT after treatment with latanoprost (mean percent change in CCT=−1.1±2.5%) and the fixed combination (mean percent change in CCT=−1.0±2.0%). These changes might be attributed to effects of PGF2α analogues on the extracellular matrix of the corneal stroma via upregulation of matrix metalloproteinases. Our series confirmed that topical bimatoprost induced a statistically significant decrease in CCT, an effect not observed with latanoprost and travoprost. However, the observed change induced by bimatoprost was clinically small (<1%), insufficient to promote significant changes in IOP measurements with Goldmann applanation tonometry.
The results of the present study suggest that bimatoprost, latanoprost, and travoprost significantly reduce the IOP of phakic eyes with POAG and OHT without causing significant changes on the BAB after 4 weeks. Although a significant reduction in mean CCT was observed when patients instilled bimatoprost for 4 weeks, this change can be considered clinically irrelevant.
References
Alm A . Prostaglandin derivates as ocular hypotensive agents. Prog Retin Eye Res 1998; 17: 291–312.
Linden C . Therapeutic potential of prostaglandin analogues in glaucoma. Expert Opin Investig Drugs 2001; 10: 679–694.
Alexander CL, Miller SJ, Abel SR . Prostaglandin analog treatment of glaucoma and ocular hypertension. Ann Pharmacother 2002; 36: 504–511.
Stamper RL, Wigginton SA, Higginbotham EJ . Primary drug treatment for glaucoma: beta-blockers versus other medications. Surv Ophthalmol 2002; 47: 63–73.
Stewart WC, Kolker AE, Stewart JA, Leech J, Jackson AL . Conjunctival hyperemia in healthy subjects after short-term dosing with latanoprost, bimatoprost, and travoprost. Am J Ophthalmol 2003; 135: 314–320.
Camras CB, Toris CB, Tamesis RR . Efficacy and adverse effects of medications used in the treatment of glaucoma. Drugs Aging 1999; 15: 377–388.
Parrish RK, Palmberg P, Sheu WP . A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol 2003; 135: 688–703.
Arcieri ES, Santana A, Rocha FN, Guapo GL, Costa VP . Blood–aqueous barrier changes after the use of prostaglandin analogues in patients with pseudophakia and aphakia: a 6-month randomized trial. Arch Ophthalmol 2005; 123: 186–192.
Lima MC, Paranhos Jr A, Salim S, Honkanen R, Devgan L, Wand M et al. Visually significant cystoid macular edema in pseudophakic and aphakic patients with glaucoma receiving latanoprost. J Glaucoma 2000; 9: 317–321.
Miyake K, Ota I, Ibaraki N, Akura J, Ichihashi S, Shibuya Y et al. Enhanced disruption of the blood–aqueous barrier and the incidence of angiographic cystoid macular edema by topical timolol and its preservative in early postoperative pseudophakia. Arch Ophthalmol 2001; 119: 387–394.
Miyake K, Ibaraki N . Prostaglandins and cystoid macular edema. Surv Ophthalmol 2002; 47 (Suppl 1): S203–S218.
Warwar RE, Bullock JD, Ballal D . Cystoid macular edema and anterior uveitis associated with latanoprost use. Experience and incidence in a retrospective review of 94 patients. Ophthalmology 1998; 105: 263–268.
Ayyala RS, Cruz DA, Margo CE, Harman LE, Pautler SE, Misch DM et al. Cystoid macular edema associated with latanoprost in aphakic and pseudophakic eyes. Am J Ophthalmol 1998; 126: 602–604.
Wand M, Shields BM . Cystoid macular edema in the era of ocular hypotensive lipids. Am J Ophthalmol 2002; 133: 393–397.
Moroi SE, Gottfredsdottir MS, Schteingart MT, Elner SG, Lee CM, Schertzer RM et al. Cystoid macular edema associated with latanoprost therapy in a case series of patients with glaucoma and ocular hypertension. Ophthalmology 1999; 106: 1024–1029.
Watanabe K, Hayasaka S, Hayasaka Y, Nagaki Y, Watanabe K . Cystoid macular edema associated with latanoprost use in a pseudophakic eye with a history of surgical complications. Jpn J Ophthalmol 2003; 47: 110–112.
Miyake K, Ota I, Maekubo K, Ichihashi S, Miyake S . Latanoprost accelerates disruption of the blood–aqueous barrier and the incidence of angiographic cystoid macular edema in early postoperative pseudophakias. Arch Ophthalmol 1999; 117: 34–40.
Furuichi M, Chiba T, Abe K, Kogure S, Iijima H, Tsukahara S et al. Cystoid macular edema associated with topical latanoprost in glaucomatous eyes with a normally functioning blood–ocular barrier. J Glaucoma 2001; 10: 233–236.
Smith SL, Pruitt CA, Sine CS, Hudgins AC, Stewart WC . The use of latanoprost 0.005% once daily to simplify medical therapy in patients with primary open-angle glaucoma or ocular hypertension. Acta Ophthalmol Scand 1999; 77: 189–192.
Cellini M, Caramazza R, Bonsanto D, Bernabini B, Campos EC . Prostaglandin analogs and blood–aqueous barrier integrity: a flare cell meter study. Ophthalmologica 2004; 218: 312–317.
Fechtner RD, Khouri AS, Zimmerman TJ, Bullock J, Feldman R, Kulkarni P et al. Anterior uveitis associated with latanoprost. Am J Ophthalmol 1998; 126: 37–41.
Widengard I, Maepea O, Alm A . Effects of latanoprost and dipivefrin, alone or combined, on intraocular pressure and on blood–aqueous barrier permeability. Br J Ophthalmol 1998; 82: 404–406.
Hotehama Y, Mishima HK . Clinical efficacy of PhXA34 and PhXA41, two novel prostaglandin F2 alpha-isopropyl ester analogues for glaucoma treatment. Jpn J Ophthalmol 1993; 37: 259–269.
Linden C, Nuija E, Alm A . Effects on IOP restoration and blood–aqueous barrier after long-term treatment with latanoprost in open angle glaucoma and ocular hypertension. Br J Ophthalmol 1997; 81: 370–372.
Viestenz A, Martus P, Schlotzer-Schrehardt U, Langenbucher A, Mardin CY . Impact of prostaglandin-F(2alpha)-analogues and carbonic anhydrase inhibitors on central corneal thickness—a cross-sectional study on 403 eyes. Klin Monatsbl Augenheilkd 2004; 221: 753–756.
Goldmann H, Schmidt T . Applanation tonometry. Ophthalmologica 1957; 134: 221–242.
Herndon LW, Weizer JS, Stinnett SS . Central corneal thickness as a risk factor for advanced glaucoma damage. Arch Ophthalmol 2004; 122: 17–21.
Shih CY, Graff Zivin JS, Trokel SL, Tsai JC . Clinical significance of central corneal thickness in the management of glaucoma. Arch Ophthalmol 2004; 122: 1270–1275.
Sawa M . Clinical application of laser flare-cell meter. Jpn J Ophthalmol 1990; 34: 346–363.
Yousufzai SY, Ye Z, Abdel-Latif AA . Prostaglandin F2 alpha and its analogs induce release of endogenous prostaglandins in iris and ciliary muscles isolated from cat and other mammalian species. Exp Eye Res 1996; 63: 305–310.
van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH . Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology 2005; 112: 1177–1185.
Lass JH, Eriksson GL, Osterling L, Simpson CV, Latanoprost Corneal Effects Study Group. Comparison of the corneal effects of latanoprost, fixed combination latanoprost-timolol, and timolol: a double-masked, randomized, one-year study. Ophthalmology 2001; 108: 264–271.
Acknowledgements
This study was supported by grant 2001/09520-0 from Fundação de Amparo à Pesquisa do Estado de São Paulo, São Paulo, Brazil. Dr Costa has received research grants from Alcon Inc., Novartis, and Pfizer in the past. The authors do not have any financial or proprietary interest in the drugs and instruments mentioned in this article
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was presented in part as a poster at the Association for Research in Vision and Ophthalmology Annual Meeting Scientific Programme; 3 May 2005; Fort Lauderdale, Fla
Rights and permissions
About this article
Cite this article
Arcieri, E., Pierre Filho, P., Wakamatsu, T. et al. The effects of prostaglandin analogues on the blood aqueous barrier and corneal thickness of phakic patients with primary open-angle glaucoma and ocular hypertension. Eye 22, 179–183 (2008). https://doi.org/10.1038/sj.eye.6702542
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702542
Keywords
This article is cited by
-
The effect of topical bimatoprost on corneal clarity in primary open-angle glaucoma: a longitudinal prospective assessment
International Ophthalmology (2022)
-
Laser flare-cell photometer: principle and significance in clinical and basic ophthalmology
Japanese Journal of Ophthalmology (2017)
-
Blood–aqueous barrier integrity in patients with Graves’ ophthalmopathy (GO), before and after rehabilitative surgery
Eye (2015)
-
Switching efficacy on intraocular pressure from latanoprost to bimatoprost in eyes with open angle glaucoma: implication to the changes of central corneal thickness
Japanese Journal of Ophthalmology (2014)
-
Randomized crossover study of latanoprost and travoprost in eyes with open-angle glaucoma
Graefe's Archive for Clinical and Experimental Ophthalmology (2012)