Abstract
Purpose
Evaluation of on axis phacoemulsification surgery through temporal incision using nondominant hand with surgeon sitting at the head end, inpatients with against-the-rule astigmatism.
Methods
Eighty eyes of 80 patients who underwent phacoemulsification through a temporal clear corneal tunnel for age-related cataract and against-the-rule astigmatism were enrolled and divided into four equal groups. In Group 1A, the surgeon was sitting at the head end for the left eye performing surgery with the left hand (nondominant hand). In group 1B, the surgeon was seated at the temporal side and surgery was performed in the left eye with dominant right hand. In group 2A, the surgeon was sitting at the head end for the right eye and performed surgery holding the phacoemulsification hand piece in his right hand. In group 2B, the surgeon sat on the temporal side of the right eye and performed phacoemulsification with his right hand. The patients were followed up on day 7, 1 month, and 3 months. Parameters evaluated included average phaco power, effective phaco time, uncorrected and best-corrected visual acuity, keratometry, intraocular pressure, surgically induced astigmatism, pachymetry, and endothelial cell counts.
Results
The phaco time and phaco power among the four groups were comparable (phaco time: P=0.368; phaco power: P=0.294). The four groups were also comparable on parameters like surgically induced astigmatism (P=0.674), change in postoperative keratometric astigmatism (P=0.584), endothelial cell loss (0.921), change in ultrasonic pachymetry (P=0.476), and intraocular pressure (P=0.942). No intraoperative or postoperative complications were observed in any of the groups. The mean uncorrected visual acuity at 3 months in group 1 was 0.723±0.21; in group 2 it was 0.756±0.21; in group 3 it was 0.748±0.22, and in group 4 it was 0.732±0.23. The best-corrected visual acuity was 0.96±0.10, 0.97±0.11, 0.95±0.13, and 0.96±0.10 in the four groups at 3 months.
Conclusion
Phacoemulsification surgery can be successfully performed with nondominant hand with a good surgical outcome. The technique gives an alternative approach where surgeon does not have to shift the position to perform on-axis phacoemulsification.
Similar content being viewed by others
Introduction
Temporal incision phacoemulsification is the preferred method of cataract surgery for surgeons who prefer ‘on-axis’ phacoemulsification as most of the eyes in cataractogenic age group have against the rule corneal astigmatism.1 Clear corneal incision on the steepest axis has a neutralizing effect on preoperative astigmatism.2 Performing surgery while sitting at the temporal side can be difficult as the surgeon cannot rest the operating hand on the patient's forehead. An arm rest or wrist rest may be required to overcome this problem. Sitting on the temporal side may also require alteration in position of the operating table, headboard, instrument trolley, microscope alignment, location of phaco machine, etc and this might increase the chance of breaching sterility in the operation theatre. The inconvenience and the risk of breach of sterility in the operation theatre can be avoided by performing temporal phacoemulsification with left hand for left eye and with the right hand for right eye.3
We performed a study to evaluate the surgical outcome by performing surgery through a temporal incision for patients with ‘against-the-rule’ astigmatism (ATR) with the surgeon seated at different positions and using different hands.
Methods
A prospective clinical trial was conducted at Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, in which 80 eyes of 80 subjects with immature senile cataract were enrolled for the study. Those patients were included in the study who had significant senile cataract with grade 2 and 3 nuclear sclerosis and ATR astigmatism of ≥0.5d with absence of other ocular pathologies. An informed consent was taken from all the patients who were enrolled in the study, and the procedures followed were in accordance with the ethical standards of the ethics committee of our institute. A detailed history was taken and preoperative evaluation of the eye was performed, which included uncorrected visual acuity and best-corrected visual acuity (BCVA) on Snellen's acuity drum, slit-lamp biomicroscopy of the anterior segment with grading of nuclear sclerosis, intraocular pressure, specular microscopy, and posterior segment evaluation by direct and indirect ophthalmoscopy after pupillary dilatation. If the cataract was very dense obscuring a clear view of the ocular fundus, B-scan ultrasonography was performed to rule out the presence of any posterior segment pathology. Keratometry was performed on Bausch & Lomb keratometer and corneal topography was performed using computer-assisted videokeratography (Eye-Sys 2000-SM-5515GP System). The axial length was measured using an A scan biometer (Appasamy associates, Chennai, India) and intraocular lens (IOL) power was calculated using SRK (Sanders, Retzlaff, Kraff) formula. Central corneal thickness was measured by ultrasonic pachymeter (HUP850). Emmetropia was aimed while prescribing the IOL power.
Eyes with ocular surface disorder, posterior segment disease likely to affect visual outcome, and endothelial cell counts <1500/mm2 were excluded from the study.
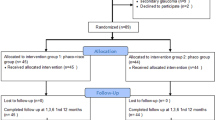
These patients were then divided into two groups depending upon the eye to be operated. Left eye was operated in group 1 (N=40), and in group 2 (N=40) surgery was performed in the right eye. Within the groups, the patients were subdivided into two sub-groups by randomisation using statistical random table (Table 1). In group 1A (20 eyes), the surgeon was sitting at the head end for the left eye and performing surgery with the left hand (non-dominant hand). In group 1B (20 eyes) the surgeon was seated at the temporal side and surgery was performed with dominant right hand. In group 2A (20 eyes), the surgeon was sitting at the head end for the right eye and performed surgery holding the phacoemulsification hand piece in his right hand. In group 2B (20 eyes), the surgeon sat on the temporal side of the right eye and performed phacoemulsification with his right hand.
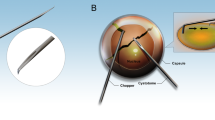
All the surgeries were performed by a single surgeon Rasik Behari Vajpayee under topical anaesthesia using 0.5% proparacaine. After aseptic cleaning and draping of the eye, a clear corneal tunnel was created in the steeper axis using a crescent blade and 3.2 mm keratome. A side port was made 2 o'clock hours to the left of the corneal tunnel except in group 1A, in which it was located to the right of the corneal tunnel, that is superiorly. Sodium hyaluronate 1.4% (Healon GV®, Pharmacia & Upjohn, Portage Road, Kalamazoo) was injected into the anterior chamber. A central curvilinear capsulorhexis was performed with a bent 26 G needle through the main tunnel when the surgeon was sitting temporally and through a 12 o'clock side port when the position of the surgeon was at the head end of the patient. After a thorough hydroprocedure, the nuclear emulsification was performed by primary chopping or by crater and chop4 technique depending upon the hardness of the nucleus by Storz protégé phaco machine (Storz Protégé, Bausch & Lomb, NY, USA). The phaco hand piece was held in the left hand in group 1A, whereas in other groups it was held in the right hand. While performing left hand phacoemulsification, the chopper was held in the right hand and the nuclear fragments were manipulated by it towards the phaco probe that was kept in the centre. A complete cortical clean up was then performed by automated irrigation and aspiration. The capsular bag was inflated with viscoelastic and a single-piece acrylic foldable IOL (ACRYSOF® SA60AT; Alcon laboratories, Fort Worth, TX, USA) was implanted in all the patients, the power of which was calculated using modified SRK II formula. The viscoelastic was completely aspirated out with the rock and roll technique and the tunnel was hydrated with balanced salt solution (BSS).
Intraoperative evaluation included effective phaco time and average phaco power.
Postoperatively, patients were prescribed topical betamethasone sodium phosphate 0.1% and ciprofloxacin 0.3% q.i.d. each for 4 weeks and tropicamide 1% t.i.d. for 1 week.
Postoperatively, all the parameters were recorded at 1 week, 1 month, and 3 months using the same method and instruments.
The data were analysed by applying analysis of variance first and then for sub-group comparison. Post hoc test (Bonferroni) was used (SPSS software). Surgically induced refractive change was calculated by using Holladay, Cravy, and Koch's formula.5
Results
The overall mean age of the patients was 62.52±9.33 years with all the four groups having a similar and statistically comparable age group (group 1A: 62.00±8.60; group 1B: 62.85±7.40; group 2A: 62.70±10.65; and group 2B: 62.55±10.67). Fifty-four (67.50%) patients were males and the male-to-female ratio between the groups were comparable. The grades of nuclear sclerosis were also comparable between the four groups (Table 2). Phacoemulsification was performed successfully in all the eyes and no intraoperative complication was noted in any of the eyes. The phaco time and phaco power among the four groups were comparable (Table 3). Surgically induced astigmatism, change in postoperative keratometric astigmatism, endothelial cell loss, change in ultrasonic pachymetry, and postoperative intraocular pressure (IOP) were also comparable at 3 months follow-up in all the groups. The mean phaco power used while performing phacoemulsification by dominant (n=60) and nondominant hand (n=20) were 17.90±3.65 and 19.18±3.22, respectively (P-value: 0.43). The mean uncorrected visual acuity at 3 months in group 1 was 0.72±0.21; in group 2 it was 0.75±0.21; in group 3 it was 0.74±0.22, and in group 4 it was 0.73±0.23 (P-value (among groups): NS). The BCVA was 0.96±0.10, 0.97±0.11, 0.95±0.13, and 0.96±0.10 in the four groups at 3 months (P-value (among groups): >0.05).
Discussion
Cataract surgery has advanced so much after the introduction of phacoemulsification that it is now regarded as an integral part of keratorefractive surgery aiming towards emmetropia. In recent times, surgeons have aimed towards providing astigmatically neutral eyes postoperatively. There are several studies that have shown that a temporal incision is more stable, neutralizes ATR astigmatism,6, 7 gives better access in deep set eyes and provides better coaxial view. However, performing surgery while sitting at the temporal side can be discomforting to the surgeon as the surgeon cannot rest the operating hand. More importantly for surgeons practising ‘on-axis’ phaco, it requires readjustment of surgeons' position between cases when operating on patients with ‘with’ or ‘ATR astigmatism’. Moreover, for high volume surgeons, this can add unnecessary inconvenience between cases as it involves adjustment of foot switch positions and rearrangement of placement of the phaco machine and instrument trolley, surgeons' seat, angulations of the operating microscope and thereby increasing the total operation theatre (OT) time as well as the increased requirement of OT staffs. If the surgeon continues to sit at the head end and uses the phacoemulsification hand piece in either hand through the temporal incision for patients with ATR astigmatism, the need for all this realignment would be eliminated. However, this approach necessitates holding the phaco probe in the nondominant hand for operating on left eyes for a right-handed surgeon. The present study was designed to study the feasibility of this approach and compare the effect of surgeons seating position and the results obtained with dominant and nondominant hand surgery through a temporal incision in patients with ‘ATR’ astigmatism.
We observed that there was no significant difference in the surgical outcome between eyes operated with dominant or nondominant hand. All the groups were comparable with respect to all the parameters evaluated, that is BCVA, IOP, specular count, pachymetry, and surgically induced astigmatism. Based on this study, we could infer that the various methods employed for temporal phacoemulsification are equivalent and either of these may be chosen as per surgeon's choice without jeopardizing visual outcome.
A previous retrospective study3 has reported comparable complication rate between nondominant and dominant hand phacoemulsification. In our study, there was no complication detected in any group and this could be attributed to surgery performed by an experienced surgeon; however, the same may not hold true if the surgery is performed by a less experienced surgeon. It has also been suggested that chopping may be easier by the dominant hand as it is technically more difficult than phaco as in the latter, the hand is relatively immobile.1
It has also been reported earlier that the overall rate of complications was lower in nondominant hand phacoemulsification (14.5%) compared to dominant hand operated eyes (19.7%), although this was not significant.3 The possible reason may be that the ultrasound hand piece that is held in the nondominant hand is kept as a passive instrument in the central part of the capsular bag and the chopper that is the active instrument used for manoeuvring inside the bag and the anterior chamber is held in the dominant hand. This helps to reduce the use of ultrasound energy for nuclear management, as the chopper is the primary instrument used for the mechanical disintegration of the nucleus.8 Moreover, if we could perform surgery by sitting at one position, that is, at head end and by changing the hand used for either eye, we could obviate the need for the surgeon to change position in between cases. This not only would help us in evaluating the concept of nondominant hand phacoemulsification but also help us in getting an insight into this new technique.
To conclude, the technique gives an alternative approach to surgery in patients with ‘ATR’ astigmatism in order to help the surgeon who prefers ‘on-axis’ phacoemulsification.
References
Leaming DV . Practice style and preferences of ASCRS members–2000 survey. J Cataract Refract Surg 2001; 27: 948–955.
Zemaitiene R, Jasinskas V, Januleviciene I . Correction of corneal astigmatism during phacoemulsification. Medicina (Kaunas) 2003; 39 (12): 1175–1183.
Kageyama T, Yaguchi S, Metori Y, Chida M, Koizumi K, Onishi T et al. Visual results and complications of temporal incision phacoemulsification performed with the non-dominant left hand by junior ophthalmologists. Br J Ophthalmol 2002; 86 (11): 1222–1224.
Vanathi M, Vajpayee RB, Tandon R, Titiyal JS, Gupta V . Crater and chop technique for safe phacoemulsification in hard cataracts. J Cataract Refract Surg 2001; 27 (5): 659–661.
Holladay JT, Cravy TV, Koch DD . Calculating the surgically induced refractive change following ocular surgery. J Cataract Refract Surg 1992; 18: 429–443.
Kohnen T, Dick B, Jacobi KW . Comparison of the induced astigmatism after temporal clear corneal tunnel incision of different sizes. J Cataract Refract Surgery 1995; 21: 417–424.
Goes Jr FM, Goes JF . Astigmatic changes after sutureless small incision cataract surgery using a superior or temporal corneal incision. Bull Soc Belg Ophthalmol 1998; 268: 27–32.
Vajpayee RB, Moulick P, Sharma N, Tandon R . Left handed non-dominant phacoemulsification. Br J Ophthalmol 2003; 87: 660.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, V., Sinha, R., Sharma, N. et al. Phacoemulsification with nondominant hand. Eye 21, 1037–1040 (2007). https://doi.org/10.1038/sj.eye.6702390
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702390
Keywords
This article is cited by
-
Plasma and aqueous levels of subfatin, preptin and betatrophin in patients with diabetic retinopathy
BMC Ophthalmology (2023)
-
Plasma and aqueous levels of alarin and adipsin in patients with and without diabetic retinopathy
BMC Ophthalmology (2022)