Abstract
Introduction
Although the lymphatic system was first described almost 400 years ago, it is only in very recent years that researchers have been able to identify lymphatic channels with reasonable accuracy. Through advances in molecular biology and the development of endothelial cell markers the long held view that the human orbit is devoid of lymphatics has now been challenged.
Discussion
This review discusses the current evidence on this topic, which confirms the presence of orbital lymphatics in lachrymal gland and optic nerve sheath.
Similar content being viewed by others
Introduction
Gasper Aselli first described the human lymphatic system in 1627.1 Its function is continuous removal of interstitial fluid and macromolecules including proteins from the extracellular space, and transport of this lymph through lymph nodes before returning it to the venous circulation. The lymphatic system thus has a prime function in immune surveillance. A better understanding of it might significantly enhance our understanding of autoimmune associated orbital diseases such as Graves' orbitopathy, and well as the spread of neoplastic disease including lymphoma, to and from the orbit.
Until the present era of enzyme histochemistry and gene expression analysis, identification of lymphatic channels has rested on histological criteria. For the smallest lymphatic capillaries, morphological identification criteria comprise thin-walled channels of fenestrated endothelium lacking a well-developed basement membrane and showing no intraluminal red blood cells. However, despite these criteria, light microscopy cannot accurately differentiate between blood and lymphatic capillaries.
Although the organization of the lymphatic system generally parallels that of the blood system, lymphatics are not distributed uniformly throughout the body. While the eyelids and bulbar conjunctiva are rich in lymphatics,2, 3 and avascular tissues such as the lens and cornea have none, several vascularised tissues, notably parts of the central nervous system, also appear devoid of lymphatics. Until very recently, the human orbit was similarly thought to lack any lymphatic system, but why should this be? The orbit is a space bound by rigid bones, and fascial boundaries that are only semidistensible. Blood capillaries are known to leak protein continuously which cannot readily be reabsorbed against its concentration gradient. Therefore, this protein rich interstitial fluid must leave the orbit and be returned to the vascular compartment if a harmful rise in intraorbital pressure is to be avoided. An alternative concept of flow via ‘prelymphatic’ spaces provides a possible model for interstitial fluid to leave tissues devoid of lymphatics and drain to adjacent lymphatics.4 However, the orbit remains a relatively large vascularised tissue space for such a model to encompass. Additionally, although adjacent lymphatics are plentiful anterior to the orbit, no lymphatics have ever been identified which could collect fluid from prelymphatics posterior to the orbit.5 Furthermore, a number of authors have proposed that part in the cerebrospinal fluid outflow pathway involves the lymphatic system, and that such a system exists in the orbit.6, 7, 8
In the context of the lymphatic identification problems already described, the issue as to whether orbital lymphatics exist continued to challenge researchers until very recently. Several approaches were taken in attempts to identify orbital lymphatic flow. One approach involved injection of substances selectively taken up by lymphatics such as macromolecule of India ink or radiolabelled colloids. Most such orbital studies involved only nonprimates, however, a study in monkeys5 suggested that while extraconal injections all drained anteriorly, intraconal injections also spread posterior and entered the contralateral orbit. This did not, however, give any specific information on orbital lymphatic channels. A second approach involved ligation of known lymphatics close to the orbit.4, 9 These studies likewise suggest orbital lymphatic drainage, but again did not specifically locate orbital lymphatics. In the last decade or so, other techniques using scanning and transmission electron microscopy have been used to improve the identification of lymphatics using morphological criteria. To qualify as a lymphatic, Sherman10 applied the following criteria: a vessel with a discontinuous basal lamina, irregular lumen and lack of the specific fenestrations seen on certain blood vessels, but with anchoring filaments to surrounding connective tissue. In addition, the endothelial cells must show extremely attenuated cytoplasm and interendothelial gaps, but lack tight junctions. In 1999, Killer11 examined human optic nerve meninges by electron microscopy following India ink injection into the subarachnoid space and demonstrated lymphatics in dura particularly in the bulbar portion of the nerve. He concluded that CSF might drain via arachnoid pores into the dural lymphatics in this area.
Recent advances in enzyme histochemistry and molecular biology have now allowed the identification of lymphatics to move away from purely histological criteria. With these techniques it is now possible to identify lymphatic endothelium with far greater accuracy and further challenge some of the long held beliefs concerning the orbital lymphatic system.
Recent advances in lymphatic identification
Enzymes studies
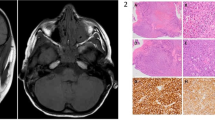
Studies from the 1970s showed differences in expression of both 5 nucleotidase (5′-Nase) and alkaline phosphatase (ALP-ase): while lymphatic endothelium expressed high levels of 5′-Nase and low levels of ALP-ase, the reverse was true for blood vessel endothelium.12, 13, 14 In 1993, these enzyme techniques were first used to study primate orbital tissue,10 and in 1999 to study human orbital tissue, including two nonirradiated exenteration specimens, and six enucleation specimens consisting of anterior optic nerve and globe.15 Using conjunctiva as a control, lymphatics were identified in the following human orbital tissues by double staining with 5′-Nase followed by ALP-ase. In each case, lymphatics were only confirmed to be present if the 5′-Nase staining vessels also met the morphological criteria for lymphatics already described. In order to improve cellular architecture, and assist this identification, plastic sections, which do not obliterate enzyme activity, were used.15 Lachrymal gland: Both orbital and palpebral lobes showed numerous lymphatic capillaries adjacent to blood vessels and lachrymal gland ductules (Figure 1). Optic nerve: Lymphatics were found in the middle and outer layers of the dura but not the optic nerve itself (Figure 2). Although 5′-Nase staining was also found in the base of the arachnoid, no vessels meeting the morphological criteria for lymphatics could be discerned. Orbital Apex: 5′-Nase incubation revealed brown staining structures that met some of the morphological criteria for lymphatics, however, as not all criteria were met, it was not possible to verify the presence of lymphatics in this area. Orbital Fat: no lymphatics were identified, however, technical difficulties may have influenced this. Extraocular muscles: although only the belly of each rectus muscle was examined, no lymphatics were found. Some findings of this human study differed from that of the earlier primate study by Sherman,10 which gave conclusive evidence of lymphatics at the orbital apex, and also suggested lymphatics might be present in extra-ocular muscles although conclusive electron microscopy evidence was lacking.
Gene expression profile analysis
Considering the differing specialized functions of the lymphatic and blood vessel systems, it is not surprising that molecular differences in their endothelial cells continue to emerge. They express distinct sets of vascular markers and respond differently to growth factors. For example, studies using growth factors have identified that whereas vascular endothelial growth factor receptors-1 and -2 (VEGF R-1, VEGF R-2) are important for blood vessel angiogenesis, VEGFR-3, a tyrosine kinase receptor for VEGF-C and VEGF-D, is important for lymphangiogenesis. Nevertheless, immunohistochemical staining techniques for many of these markers have not been able to distinguish reliably between blood and lymphatic capillaries. In the last few years, however, a selective lymphatic marker has emerged to improve lymphatic identification significantly.16 It is a monoclonal antibody, D 2-40, which reacts to an Mv 40 000 O-linked sialoglycoprotein on lymphatic endothelium. Additionally, it is suitable for fixed tissue specimens without epitope retrieval thus permitting far better preservation of cellular architecture. Using a monoclonal antibody that is specific for blood vessel endothelium, CD-34, plus D2-40, lymphatics were again confirmed in human lachrymal gland where they were abundant (Figure 3), and optic nerve dura, where they were few.17 Another study examined human retrobulbar adipose tissue from 10 orbits using monoclonal antibody CD-34 and D2-40 differentiation of blood and lymphatic vessels.18 While no lymphatics were identified in normal anterior orbital fat, three of the orbits showing areas of inflammation also showed areas of positive D2-40 staining, although not as distinct endothelial cell-lined vessels. These findings suggest the possibility of inflammation-induced vessel formation. Similar findings have been observed in experimental wounds which show that lymphangiogenesis parallels angiogenesis in granulation tissue formation.19
Discussion
The search for orbital lymphatics is of great importance: while it may significantly enhance our understanding of the spread of neoplasia, it may also improve our understanding of more common orbital pathologies. Of great importance is the mechanism by which orbital oedema resolves. Within the confined space of the orbit, the volume increase associated with inflammation (as in Graves' orbitopathy), can potentially induce a sight threatening pressure rise unless either the orbit expands (to give proptosis) or the interstitial fluid is able to leave the orbit. The recent work on inflammation-associated lymphangiogenesis19 may be beginning to shed light on this process, although the actual exit of extravascular fluid from the orbit is far from clear. The origin of the cells destined to develop into lymphatic endothelium is also not yet clear. Some evidence supports a pluripotent stem cell progenitor in that the transcription factor Prox-1 has been shown to induce lymphatic endothelium to develop from blood vascular endothelium.20
Another issue yet to be resolved concerns the origin of orbital lymphangiomas. These hamartomas contain vessels that fit morphological criteria for lymphatics. Furthermore, a recent study using D2-40 has confirmed lymphatic endothelial channels in cutaneous and intestinal lymphangiomas.21 Hamartomas generally develop from cells already present at their site of origin, however, this does not necessarily imply the existence of mature lymphatic endothelial cells within the prenatal orbit, and most authors have concluded that a pluripotent stem cell progenitor is more likely.22
A third area of interest concerns the role of dural lymphatics in the absorption of cerebrospinal fluid (CSF) from the optic nerve meninges under normal conditions and in the presence of raised CSF pressure.8, 11 Further understanding in this area will shed light on phenomena such as unilateral papilloedema and pseudotumour cerebri.
Conclusions
It is now abundantly clear that lymphatics are present in at least some areas of the human orbit. In the light of recent advances in vascular markers, it is likely that studies in the near future will be able to prove whether or not other orbital tissues such as the extraocular muscles and posterior orbital fat also possess a lymphatic system. As our knowledge of lymphatic anatomy improves, so too will our presently limited appreciation of orbital lymphatic physiology.
References
Clodius L . Lymphoedema. In: McCarthy JG (ed). Plastic Surgery, Vol 6: the Trunk and Lower Extremity. Philadelphia: W.B. Sunders, 1990, pp 4093–4101.
Gusev AM . Lymph vessels of human conjunctiva. Arkh Anat Gistol Embriol 1963; 45: 1099–1102.
Gusev AM . Intraorgan lymphatic bed of eyelids in man. Arkh Anat Gistol Embriol 1967; 9: 93–95.
Casley-Smith JR, Födi-Boresok E, Földi M . The prelymphatic pathways of the brain as revealed by cervical lymphatic obstruction and the passage of particles. Br J Exp Path 1976; 57: 179–188.
McGetrick JJ, Wilson DG, Dortzbach RK, Kaufman PL, Lemke BN . A search for lymphatic drainage of the monkey orbit. Arch Ophthalmol 1989; 107: 255–260.
Bradbury MWB, Cole DF . The role of the lymphatic system in the drainage of cerebrospinal fluid and aqueous humor. J Physiol 1980; 299: 353–365.
Ehrlich SS, McComb J, Hyman S . Ultrastructure of the orbital pathway for cerebrospinal fluid drainage in rabbits. J Neurosurg 1989; 70: 926–931.
Brinker T, Lüdemann W, Berens von Rautenfeld D . Dynamic properties of the lymphatic pathways for the absorption of cerebrospinal fluid. Arch Neuropathol 1997; 94: 493–498.
Casley-Smith JR, Clodius L, Foldi-Boresok E, Grüntzig J, Földi M . The effects of chronic cervical lymphostasis on regions drained by lymphatics and by pre-lymphatics. J Pathol 1978; 124: 13–17.
Sherman DD, Gonnering RS, Wallow IHL, Lemke BN, Doos WG, Dortzbach RK et al. Identification of orbital lymphatics: enzyme histochemical light microscopic and electron microscopic studies. Ophthal Plast Reconstr Surg 1993; 9 (3): 153–169.
Killer HE, Laeng HR, Groscurth P . Lymphatic capillaries in the meninges of the human optic nerve. J Neuro-ophthalmol 1999; 19 (4): 222–228.
Vetter W . Alkalische phosphatasen in mastzellen, blut und lymphgefässen der rattenzunge: 5′-nucleotidase-, unspecifische alkalische phosphatase- und polyphosphatase- (ATP'ase) aktivität unter besonderer berücksichtigung des pH. Z Anat Entwickl Gesch 1970; 130: 153–176.
Heusermann U . Morphologie der lymphgefässe, der nerven, der kapsel, und der trabekel des menschen. Kiel: Milz Habilschr Med Fakultat 1979.
Kato S . Histochemical localization of 5′-nucleotidase in the lymphatic endothelium. Acta Histochem Cytochem 1990; 23: 613–620.
Gausas RE, Gonnering RS, Lemke BN, Dortzbach RK, Sherman DD . Identification of human orbital lymphatics. Ophthal Plast Reconstr Surg 1999; 15 (4): 252–259.
Kahn HJ, Bailey D, Marks A . A monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol 2002; 15: 434–440.
Gausas RE . Advances in applied anatomy of the eyelid and orbit. Curr Opin Ophthalmol 2004; 15: 422–425.
Fogt F, Zimmerman RL, Daly T, Gausas RE . Observation of lymphatic vessels in orbital fat of patients with inflammatory conditions: a form fruste of lymphangiogenesis? Int J Mol Med 2004; 13: 681–683.
Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K . Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol 2000; 156: 1499–1504.
Partanen TA, Paavonen K . Lymphatic versus blood vascular endothelial growth factors and receptors in humans. Microsc Res Tech 2001; 55 (2): 108–121.
Fukunaga M . Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology 2005; 46: 396–402.
Harris GJ, Sakol PJ, Bonavolontà G, De Conciliis C . An analysis of thirty cases of orbital lymphangioma. Ophthalmol 1990; 97: 1583–1592.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dickinson, A., Gausas, R. Orbital lymphatics: do they exist?. Eye 20, 1145–1148 (2006). https://doi.org/10.1038/sj.eye.6702378
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702378
Keywords
This article is cited by
-
Zygomaticomaxillary complex fractures: finding the least complicated surgical approach (A Randomized Clinical Trial)
BMC Oral Health (2023)
-
Orbital venous pattern in relation to extraorbital venous drainage and superficial lymphatic vessels in rats
Anatomical Science International (2017)
-
Conjunctival oedema as a potential objective sign of intracranial hypertension: a short illustrated review and three case reports
Acta Neurochirurgica (2013)