Abstract
Purpose
To prospectively assess the efficacy of bimanual phacoemulsification and implantation of Thinoptx®, an injectable intraocular lens (IOL), inserted through 1.70 mm clear corneal incision.
Setting
Department of Ophthalmology, Southend Hospital NHS Trust, UK.
Methods
A total of 50 eyes of 49 randomly selected patients with cataracts had microincision clear corneal bimanual phacoemulsification (MICS) with implantation of Thinoptx® IOL in the capsular bag. All patients underwent full preoperative assessment. Postoperative assessment was carried out at 3 and 6 weeks and at 15 months.
Results
In all 50 cases the IOL was inserted through 1.70 mm clear corneal incision. The mean best-corrected visual acuity was 0.02 (6/6-1) at 6 weeks and was 0.17 (6/10) at the final follow-up. The mean final surgically induced astigmatism at 6 weeks was 0.0106. Coloured haloes around artificial lights were perceived by 69.23% of patients at 6 weeks and by 61.29% at the final follow-up. One patient underwent IOL exchange for this. Posterior capsular opacification was noticed in 31.26% at 6 weeks and in 64.51% at 15 months. Anterior capsular opacification was noticed in 5.26% at 6 weeks and in 16.12% at 15 months. In one patient the IOL had to be exchanged because of tilt and displacement of the IOL due to anterior capsular phimosis.
Conclusions
We conclude Thinoptx® can be safely inserted through 1.70 mm incision used for bimanual phacoemulsification. Distance and near visual acuity achieved with this IOL is satisfactory. There is no significant change in keratometric astigmatism following this procedure. However, posterior capsular opacification rate was significantly higher with this IOL. Haloes around light sources were significant.
Similar content being viewed by others
Introduction
The aim of modern cataract surgery is rapid restoration of vision and low incidence of complications and postoperative refractive errors. Bimanual microincision phacoemulsification (microincision clear corneal bimanual phacoemulsification (MICS)) has been a potential technique for a number of years.1 The advantages of MICS have been a source of interest for cataract surgeons and intraocular lens (IOL) manufacturers who are now developing technologies that will permit the introduction of lens implants through sub-2 mm incisions. The main disadvantage of MICS remains the lack of IOLs that can fit through microincisions, necessitating the enlargement of wounds for IOL implantation. A recent addition to the IOLs is the Thinoptx® lenses, which can be inserted through 1.7 mm, clear corneal incision. In this study, we prospectively assessed the clinical and visual outcomes of implantation of this lens following bimanual phacoemulsification.
Materials and methods
In all, 50 eyes of 49 patients randomly selected with cataracts had MICS with implantation of injectable Thinoptx® IOL in the capsular bag.
Preoperatively
All patients had assessment of uncorrected and best-corrected distance visual acuity and refraction. None had ocular abnormality other than cataract. All grades of cataract were included in the study.2
Exclusion criteria were anterior segment pathology such as chronic uveitis, psuedoexfoliation syndrome, advanced visual field defects due to glaucoma, diabetic retinopathy, age related macular degeneration, and previous ocular surgery.
Postoperatively
Patients were reviewed at 3 and 6 weeks and 15 months following surgery. Uncorrected and best-corrected distance visual acuity was assessed with snellens (logMAR) Uncorrected and best-corrected visual near visual acuity was measured with royal college of ophthalmologist reading chart.
Slit-lamp biomicroscopy, Goldmann's applanation intraocular pressure, and fundoscopy were performed preoperatively and at 3 and 6 weeks and 15 months following surgery.
Magnitude of astigmatic change was also assessed at 6 weeks. Postoperative corneal curvature was measured with the IOL master® and mean final surgically induced astigmatism was calculated by simple subtraction.3
The Thinoptx® IOL
Ultra choice 1.0 Thinoptx® IOL (Thinoptx Inc.) is a posterior chamber IOL of hydrophilic acrylic material. The overall length of the lens is 11.2 mm, the optic is 350 μm, and haptic footplates are as thin as 50 μm (Figure 1). This is in contrast to most other hydrophilic acrylic IOLs, which have a central thickness from 1.0 to 1.2 mm depending on the lens power.4 The ultrathin lens is manufactured by lathe cutting one surface to retain a continuous curvature while the second surface is maintained within micrometres of the opposite surface. A series of steps 50 μm in height are lathe cut into the second surface to keep the lens thin. Each step can, therefore, be focused on a single point, which should eliminate spherical aberration. As a result of this lens design, the implant can be folded or rolled to be inserted through an ultrasmall incision (1.7 mm) curvature.
The thinness is one reason that thinoptx IOL can be measured in 1/8 diopter increments5 (Figure 2).
Surgical procedure
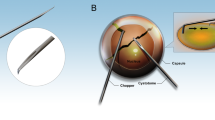
All operations were performed by two surgeons. Topical anaesthesia (proxymethacaine 0.5% eye drops) was administered twice 10 min apart before surgery. Two clear corneal incisions, 1.5 mm, were created at 12 O'clock and 2 O'clock with a ‘sharp point®’ 1.5 mm trapezoid knife (Figure 3) Viscoelastic material was injected, and a continuous curvilinear capsulorrhexis with a diameter of approximately 5 mm was created with a bent 26-gauge needle. After gentle hydrodissection, bimanual phacoemulsification was performed using white star micropulse technology (Soverign AMO®) with sleeveless 20-gauge phacotip and an irrigation chopper.6, 7 Irrigation and aspiration were performed bimanually using separate disposable cannulae for irrigation and aspiration (Figure 3). Capsular bag was filled with viscoelastic material and the 12 O'clock incision was then enlarged to 1.7 mm.
The Thinoptx® IOL was loaded in its injector and inserted into the bag (Figures 2, 5, 6, 7 and 8). The teardrops fenestration holes in the plate haptics were left pointing in the clockwise direction to ensure correct orientation of the implant. The viscoelastic material was removed from the capsular bag and the anterior chamber with irrigation and aspiration cannulae and the entry sites were closed by stromal hydration using BSS.
All eyes received topical chloramphenicol and dexamethasone 0.1% for 1 month after surgery.
Results
Intraoperative findings
In all the 50 cases the IOL was inserted through 1.70 mm clear corneal incision. It remained well-centred intraoperatively after insertion.
In three eyes the IOL had to be replaced with another Thinoptx® because of damaged lens (tear) noticed intraoperatively after insertion.
At 3 weeks
In all the 50 eyes the IOL remained well centred. The mean uncorrected distance visual acuity was 6/10 (0.2) and the mean best-corrected visual acuity was 6/8 (0.1) (Figure 4). Routine ophthalmologic examination did not reveal any anterior segment or fundus pathology.
At 6 weeks
The mean uncorrected distance visual acuity was 6/8 (0.1) (Figure 4) and the mean best-corrected distance visual acuity was 6/6-1 (0.02) (Figure 4). Near vision was n/6 or better with a correction of 2.50±0.50 D. The mean final induced astigmatism was 0.0106.
Haloes around bright lights especially in dim light were noticed by 69.23% of patients. Two patients were started on plilocarpine 2% eye drops when reviewed at 6 weeks.
Posterior capsular opacification was noticed in 31.26% at 6 weeks. Areas of interest were the total IOL optic, the central 3-mm zone as well as the capsulorrhexis.
At 15 months
A total of 31/50 (62%) patients were followed up at this final follow-up.
The mean UCVA was 0.29 (6/12-1) and the mean BCVA was 0.17(6/10-2).
Near vision was n/8 or better with correction of 2.50±0.25.
Perception of coloured haloes around light sources though not troublesome was noticed by 61.29% of the followed up patients. However, one patient underwent IOL exchange for this.
In all, 29.03% had already undergone YAG capsulotomy and a further 35.48% was advised YAG capsulotomy at this follow-up. In total, 64.51% had significant PCO necessitating YAG capsulotomy (Figure 9).
Anterior capsular opacification was noticed in 16.12% (5/31) at 15 months (Figure 10). In one patient the IOL had to be exchanged because of tilt and displacement of the IOL due to anterior capsular phimosis.
Discussion
Evolution of microincision cataract surgery
In the prephacoemulsification era, the focus in cataract surgery was in easy removal of the nucleus and hence incisions as large as 160–170°were made to facilitate this. The advent of phacoemulsification by Kelman in 1967 was a major milestone in reducing the incision sizes in modern cataract surgery. However, they had to be enlarged for IOL insertion. Yet another major breakthrough was the introduction of foldable silicone/hydrogel IOL in 1984 by T Mazzock and Edward Epstein.6 Eversince, surgeons and the different IOL manufacturers have come out with different techniques and technologies to make microincision cataract surgery a possibility.
A smaller incision is better to minimize intraoperative and postoperative complications. In a recent study, Tsuneoka et al6, 7 used a sleeveless phacotip to perform bimanual phacoemulsification in 637 cataractous eyes through 1.4 mm incision. However, the incision had to be widened to 2.2 mm for IOL insertion.
In this study, we report 50 cataractous eyes in which bimanual phacoemulsification was performed with insertion of the lens (Thinoptx®) through a 1.7 mm incision. It was found that intraoperatively, the lens with its injector was easy to inject and manoeuvre through a 1.70 mm clear corneal incision.8, 9 We had three cases in which the lens had to be exchanged because of damage caused during insertion. They were easily removed through slightly enlarged incision and were replaced with another Thinoptx lens without any complications.
The visual outcome achieved with this lens in terms of distance and near visual acuity was quite satisfactory. The decrease in mean uncorrected and best-corrected visual acuity reported at 15 months was due to posterior capsular opacification which improved significantly after YAG capsulotomy.10
Anterior and posterior capsule opacifications are frequent complications of foldable IOL implantation and are related to factors such as IOL material, its design,11 and surgical technique.12 Auffarth et al13 reported the rate of Nd : YAG laser capsulotomy over 3-year follow-up period to be (7.1%) in the hydrophobic acrylic group, followed by silicone (16.2%), PMMA (19.3%) and hydrophilic acrylic (31.1%), respectively. Our study shows a significantly higher incidence, 64.51% of PCO. Thinoptx being a hydrophilic acrylic lens is expected to have a higher PCO rate when compared to other hydrophobic lenses. The assessment in our study was based on clinical evaluation. We did not evaluate PCO using software and other automated techniques. Hence, we recommend further clinical and histopathological studies for objective and subjective assessment of PCO and its relation to lens design.
In all, 69.23% of patients reported coloured haloes around light sources, especially street lights. We commenced two patients on piocarpine 2% eye drops at 6 weeks since it interfered with their night driving and television viewing. A total of 61.29% continued to perceive haloes around artificial lights especially around street lights at 15 months. One of them underwent IOL exchange for this.
In a recent study on the early visual results with Thinoptx, Dogru et al4 had reported glare in two out of eight patients in the early postoperative period. Another Romanian study also reports glare.9 With the Thin Lens technology each ring has an exposed edge of 50 μm or less. On average there are three rings per lens plus the outer edge of the lens. It would seem logical to think that glare is possibly due to the light hitting the edge of the lens. We did not perform glare test in our study and we think it is essential to perform that to assess the magnitude of this problem.
The surgically induced astigmatism with the bimanual phacoemulsification14 and thinoptx lens was insignificant when assessed at 6 weeks and this is because of the small incision required for its implantation.3 However, vector analysis is a better method of assessing surgically induced astigmatism.15
Conclusions
We conclude Thinoptx® can be safely inserted through 1.70 mm incision used for bimanual phacoemulsification. Distance and near visual acuity achieved with this IOL is quite satisfactory. There is no significant change in keratometric astigmatism following this procedure. There appears to be significant glare and posterior capsule opacification with this lens. However, further studies and methods of evaluation will be needed with more patients to ascertain the extent of the problems and draw definitive conclusions.
References
Paul TA, Braga-Mele RB . Bimanual microincisional phacoemulsification: the future of cataract surgery? Curr Opin Ophthalmol 2005; 16 (1): 2–7.
Emery JM . Phakoemulsification—cataract surgery of the future? Tnt Ophthalmol Clin 1978; 18 (2): 155–170.
Ermis SS, Inan UU, Ozturk F . Surgically induced astigmatism after superotemporal and superonasal clear corneal incisions in phacoemulsification. J Cataract Refract Surg 2004; 30 (6): 1316–1319.
Dogru M, Honda R, Omoto M, Fujishima H, Yagi Y, Tsubota K et al. Early visual results with the rollable ThinOptx intraocular lens. J Cataract Refract Surg 2004; 30 (3): 558–565.
Agarwal A, Agarwal A, Agarwal S, Narang P, Narang . Phakonit: phacoemulsification through a 0.9 mm cornea! Incision. J Cataract Refract Surg 2001; 27: 1548–1552.
Tsuneoka H, Hayama A, Takahama M . Ultrasmahl-incisxon bimanual phacoemulsification and AcrySofSA3OAL implantation through a 2.2 mm incision. J Cataract Refract Surg 2003; 29: 1070–1076.
Linebarger U, Hardten DR, Shah GK, Lindstrom RL . Phacoernulsification and modern cataract surgery. Surv Ophthalmol 1999; 44: 123–127.
Pandey SK, Werner L, Agarwal A, Lal V, Patel N . Phakonit: cataract removal through a sub-1.0 mm incision and implantation of the ThinOptx rollable intraocular lens [letter]. J Cataract Refract Surg 2002; 28: 1710–1713.
Bordeianu CD . Thinoptix implant—the last border in modern cataract surgery [Article in Romanian]. Ophthalmologia 2005; 49 (1): 58–66, Sectia de Oftalmologie, Spitalului Judetean de Urgenta Prahova. BORDEIANU@RDSLINK.RO.
Buehl W, Sacu S, Findl O . Association between intensity of posterior capsule opacification and visual acuity. J Cataract Refract Surg 2005; 31 (3): 543–547.
Nishi O, Nishi K, Osakabe Y . Effect of intraocular lenses on preventing posterior capsule opacification: design versus material. Cataract Refract Surg 2004; 30 (10): 2170–2176.
Aykan U, Bilge AH, Karadayi K, Akin T . The effect of capsulorhexis size on development of posterior capsule opacification: small (4.5 to 5.0 mm) versus large (6.0 to 7.0 mm). Eur J Ophthalmol 2003; 13 (6): 541–545.
Auffarth GU, Brezin A, Caporossi A, Lafuma A, Mendicute J, Berdeaux G, et al., European PCO Study Group. Comparison of Nd: YAG capsulotomy rates following phacoemulsification with implantation of PMMA, silicone, or acrylic intra-ocular lenses in four European countries. Ophthalmol Epidemiol 2004; 11 (14): 19–29.
Paul T, Braga-Mele R . Bimanual microincisional phacoemulsification: the future of cataract surgery? Curr Opin Ophthalmol 2005; 16 (1): 2–7, Review. PMID: 15650574 [PubMed - indexed for MEDLINE].
Ermis SS, Inan UU, Ozturk F . Surgically induced astigmatism after superotemporal and superonasal clear corneal incisions in phacoemulsification. J Cataract Refract Surg 2004; 30 (6): 1316–1319, sametermis@hotmail.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented as poster at XXII ESCRS Congress in Paris in September 2004
Financial interest: Nil
Rights and permissions
About this article
Cite this article
Prakash, P., Kasaby, H., Aggarwal, R. et al. Microincision bimanual phacoemulsification and Thinoptx® implantation through a 1.70 mm incision. Eye 21, 177–182 (2007). https://doi.org/10.1038/sj.eye.6702153
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702153
Keywords
This article is cited by
-
Visual and refractive outcomes of new intraocular lens implantation after cataract surgery
Scientific Reports (2022)
-
Hyperopic shift caused by capsule contraction syndrome after microincision foldable intraocular Lens implantation: case series
BMC Ophthalmology (2019)
-
Early clinical outcome with a new monofocal microincision intraocular lens
International Ophthalmology (2016)
-
Aberrationskorrigierte Intraokularlinse für die mikroinzisionale Kataraktchirurgie (MICS)
Der Ophthalmologe (2009)