Abstract
Purpose
To report clinical, pathological, and laboratory analyses of two cases of single-piece hydrophobic acrylic intraocular lenses (IOLs), which presented with significant surface deposits during implantation.
Methods
The lenses were implanted with the manufacturer's recommended injector (loaded with Viscoat® and Healon GV®, respectively). Immediately after injection into the anterior chamber, areas on the lenses' surfaces were covered by deposits, which could not be entirely removed by irrigation/aspiration. The lenses were explanted and replaced with lenses of the same design. They underwent gross analyses, light microscopy, scanning electron microscopy, and energy dispersive X-ray spectroscopy for analysis of the elemental composition of the deposits. Liquid chromatography/mass spectroscopy was also performed to identify the presence of proteins.
Results
The deposits on the first lens had a granular appearance, forming a homogeneous layer mostly on the posterior lens surface. Larger crystal-like deposits were present mostly on the anterior surface of the second lens. Elemental analyses of the deposits in both cases revealed the presence of peaks of sodium, chloride, phosphate, and potassium, in addition to the peaks of carbon and oxygen (normal constituents of the lens material). Only protein components normally found in the anterior chamber during surgery, such as haemoglobin and albumin, were identified.
Conclusions
The results obtained suggest that the deposits in both cases may have resulted from crystallization of the ophthalmic viscosurgical device normally used during the loading of the IOLs into the cartridges.
Similar content being viewed by others
Introduction
The AcrySof® SA60AT intraocular lens (IOL) (Alcon Laboratories, Fort Worth, TX, USA) is a hydrophobic single-piece acrylic lens with an optic size of 6.0 mm and an overall length of 13.0 mm which can be inserted via the Monarch II® injector (Alcon Laboratories, Fort Worth, TX, USA).1
We report two cases of patients with age-related clinically significant cataracts, who, following uncomplicated phacoemulsification with a posterior chamber SA60AT IOL implantation, were found to have deposits on the lens. This material could be partially but not entirely removed with irrigation and aspiration. These lenses were explanted during the same procedure and another SA60AT lens was implanted into the capsular bag uneventfully. The lenses were sent to our laboratory for analyses in an attempt to ascertain the nature of their deposits.
Case 1
The patient was a 65-year-old male who underwent phacoemulsification of a 2+ nuclear sclerotic cataract with anterior cortical changes. Capsulorhexis was performed under Viscoat® (Alcon Laboratories, Fort Worth, TX, USA). Provisc® (Alcon Laboratories, Fort Worth, TX, USA) was then injected into the eye, and a SA60AT, +21.5 diopter lens was injected into the capsular bag using the Monarch II® delivery device with a ‘B’ cartridge. The lens had been loaded into the injector with Viscoat®. The physician noted that it was difficult injecting the lens into the eye with a ‘crunching sound’. Upon insertion, a white, granular material was noted immediately on the lens and in the anterior chamber. Attempts to remove this material by irrigation and aspiration were only partially successful. The lens was then cut in half with Vannas scissors and removed, under Provisc. Vigorous irrigation and aspiration was used to remove the remaining ophthalmic viscosurgical device (OVD) and the white, granular material from the capsular bag. Some of this material was noted in the clear corneal wound, which could not be removed. One half of the lens and the cartridge were sent for culture, and the other half was sent to our laboratory in a dry state for further analysis.
Another SA60AT lens was then injected into the bag, uneventfully. The wound was hydrated with balanced salt solution (BSS®, Alcon Laboratories, Fort Worth, TX, USA), subconjunctival clindamycin, and cefazolin were injected, and Muro 128® ointment (Bausch & Lomb Pharmaceuticals, Inc., Tampa, FL, USA) was placed on the eye. The patient also received an injection of levofloxacin into the anterior chamber, and was given oral levofloxacin for 10 days, which was to be started immediately upon leaving the hospital. Postoperatively, the patient initially developed corneal oedema at 1 week with BCVA at 20/200. The culture of the lens and the cartridge grew Bacillus species. At 1 month postoperatively, the last time the patient was seen, the eye was quiet, the corneal oedema had resolved, and BCVA was 20/30.
Case 2
The second case concerned a 64-year-old female patient who underwent phacoemulsification of a visually significant nuclear sclerotic cataract with implantation of the single-piece AcrySof model SA60AT lens. Healon GV® (AMO, Santa Ana, CA, USA) was the OVD used during the loading of the IOL in the Monarch II® injector with a ‘B’ cartridge. Intraoperatively, the surgeon noted the presence of a ‘crust’ on one of the haptics and the optic. It was not possible to remove this material from the IOL surface by irrigation/aspiration. The lens was bisected for explantation, and exchanged for another lens. The explant was then forwarded to our laboratory in the dry state for further analysis. The patient was last seen at the 6-month visit with 20/25 uncorrected visual acuity. Slit lamp examination was unremarkable at that time.
Analyses
One half of both lenses were received in a contact lens case in the dry state. Gross (macroscopic) analysis of the explanted IOLs was performed and gross pictures were taken using a digital camera (Nikon Camera Model D1X with a Nikon ED28-70 multifocal lens, Nikon, Tokyo, Japan). Both lenses were then microscopically evaluated and photographed under a light microscope (Olympus, Optical Co. Ltd, Tokyo, Japan). The explanted lenses were further analysed at the Electron Microscopy Center at the University of South Carolina (Columbia, SC, USA), by D Zhao, PhD. The explants were air-dried at room temperature for 3 days, mounted on a carbon sticky tape on a round sample stub for imaging analysis (without coating) using an environmental scanning electron microscope (FEI Quanta 200 ESEM, Hillsboro, OR, USA) equipped with an energy dispersive analysis of X-ray (EDAX) detector with light element capabilities.
Finally, analysis for the presence of proteins on the surface of the lenses was performed at the University of Utah Mass Spectrometry Core Facility, by CC Nelson, PhD, and P Krishna, PhD. This was performed by enzymatic digestion with trypsin followed by analysis of the resulting peptides by liquid chromatography/mass spectrometry (LC/MS). Identification of proteins was based on protein database searching using peptide molecular weight and sequence information obtained by LC/MS. Lens samples were taken from areas that did not include the deposits in question as controls, as well as from areas containing the unknown material in both cases.
Results
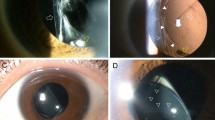
Gross examination of the lens in case 1 revealed the presence of a white granular material mostly on the posterior surface of the optic and the haptic (Figure 1a). Microscopic analysis showed the material to have a fine homogenous appearance and a brown colour (Figures 1b–d). Scanning electron microscopy confirmed the fine, granular, and homogenous appearance of the material on the posterior lens surface (Figure 2). Energy dispersive X-ray spectroscopy of the material showed a composition of sodium, phosphorus, chlorine, and potassium (Figure 3). LC/MS showed only keratin in the areas that did not have the material, which was likely a contaminant. Areas that clinically had the deposited material showed major hits for beta and delta chains of haemoglobin, and minor hits for albumin.
Gross (a) and light microscopic photographs (b–d) from the lens in case number 1. The white material noted by the surgeon intraoperatively was found to be located mostly on the posterior surface of the lens, at the optic periphery and the haptic (a). Under light microscopy (b) the material exhibited a brownish colour. The photomicrographs in (c and d) correspond to the areas delineated in (a). Note the linear marks on the lens surface (arrow in c), which could have been created during the surgeon attempts to clean the lens surface (b–d: Original magnification × 20, × 200, and × 100, respectively).
Energy dispersive X-ray spectra from the posterior surface of the lens in case number 1. The spectrum in (a) was obtained at the level of a ‘crystal-like’ fern deposit, while the spectrum in (b) was obtained at the level of the material shown in Figure 2b.
Gross surface examination of the lens in case 2 showed the presence of a grey material mostly on the anterior optic and haptic surface (Figure 4a). Small amounts of this material were also present on the posterior IOL optic surface and posterior haptic surface. Microscopic examination of the specimen showed the material to be composed of large deposits with a crystal-like appearance (Figures 4b–d). The crystals exhibited birefringence under polarized light (Figure 4d), and the crystal-like appearance of the deposits was confirmed by scanning electron microscopic examination (Figure 5). Energy dispersive X-ray spectroscopy showed the material to be composed of sodium, chlorine, and potassium (Figure 6). LC/MS showed only keratin contamination on the lens.
Gross (a) and light microscopic photographs (b–d) from the lens in case number 2. The greyish material noted by the surgeon intraoperatively was found to be located mostly on the anterior surface of the lens, at the central part of the optic, and the haptic (a). Note in (b) that the material is spread in a linear fashion on the lens surface. This pattern may have been created by the surgeon's attempts to clean the lens surface. In this case, the material was composed of large crystals (c), which exhibited birefringence under polarized light (d) (b–d: Original magnification × 100, × 200, and × 200, respectively).
Other deposits with a more fern-like appearance (probably resultant from the crystallization of salt/OVD solutions used during the explantation procedures) were also seen on both of the explanted IOLs. No bacteria were found adhering to the surfaces of the IOLs under scanning electron microscopic analyses in both cases.
Discussion
The solid extended haptics of the single-piece AcrySof® are more flexible than the traditional three-piece design. They are thus less likely to break or be permanently deformed when inserted through an injector. Injection of the entire single-piece AcrySof® in the capsular bag, without any contact of the lens with external ocular tissues, can be accomplished with the Monarch II® system. Davison's1 review of the clinical performance of the SA30AL and SA60AT lenses in 2002 concluded that these single-piece acrylic lenses performed well in all regards. A rare complication noted by the author was an ‘imperfection or deposit on the central lens optic that could not be removed from its surface, or a surface abrasion caused during the injector loading process’.
Jensen et al2 in 1994 were the first to describe crystalline deposits on the surface of silicone and poly(methyl methacrylate) (PMMA) IOLs during cataract surgery. The authors hypothesized that the phosphate in OVD preparations reacted with calcium from irrigating solutions or the aqueous humour of the patients and precipitated on the lenses. Other hypotheses included coring of syringe membranes, interactions with detergent remnants, undissolved sodium hyaluronate, or a reaction between sodium hyaluronate and the lens itself. In 1998, Olson et al3 reported the occurrence of IOL crystallization intraoperatively in 22 of 29 609 patients (0.07%) who underwent cataract surgery. This rare occurrence of crystallization was thought to be due to an osmotic gradient around the IOL, made by the OVD, resulting in increased calcium concentration and precipitation on silicone lenses. Other adverse events related to OVDs in general include precipitation formation in the solution, precipitation on the cornea, increased intraocular pressure, and intraocular inflammation.4, 5, 6
The deposited material noted in our cases was observed immediately after IOL injection, and in case 1 was thought to have been present in the injector with the IOL and OVD even before insertion in the eye (difficulty in pushing onto the injector, noted by the physician). According to Alcon, there are several steps in the manufacture of the single-piece lenses where they are individually inspected. These include gross and microscopic inspections (Mike Smith, Director of Global Marketing, personal communication, November 2003). Therefore, it is unlikely that the deposits were present on the surfaces of the lenses before manipulations for surgery.
The culture of the lens and cartridge in the first case was positive for Bacillus species. Bacillus species have been reported to occur after trauma, but also after glaucoma and cataract surgery, involving contaminated irrigating solutions or OVDs.7, 8, 9, 10, 11, 12 Culture of the OVD used to load the lens in the first case described here was not performed. In any event, with intra- and postoperative antibiotic therapy, the postoperative course of the case was uneventful, and only corneal oedema was observed during the first postoperative week.
Surface analyses performed on the material observed intraoperatively by the surgeons only demonstrated the presence of elements that may be normal components of different OVD preparations. Calcium was not found on the surface analyses. LC/MS analyses demonstrated the presence of normal protein components of the anterior chamber found during surgery, such as haemoglobin and albumin. Hypothetically, although the analyses performed were not conclusive, OVD precipitation might have occurred in the cases reported here under different circumstances, after lens loading into the injector, or immediately after injection of the lens into the eye. This precipitation should be differentiated from any crystallization of residual OVDs used during the explantation procedures. Therefore, we analysed the areas with deposits corresponding to the intraoperative observations of the surgeons, and confirmed by photographs from the explanted lenses. In such cases of intraoperative precipitation, residues from cartridges coating are also a possibility. However, the nature of the coating used by the manufacturer is proprietary information at this point.
Occurrence of OVD drying out and precipitation on the lens while it is still inside the cartridge is in theory possible. In this case, the differences in the morphology of the deposited material in both cases may have been related to the differences in the composition of the OVD used to load the lenses (3.0% sodium hyaluronate, 4.0% chondroitin sulphate in Viscoat®; 1.4% sodium hyaluronate in Healon GV®). Hyaluronan and its related anionic polysaccharides have been known to precipitate with cationic detergents. In addition, cationic proteins have also been known to cause the precipitation of anionic hyaluronan. In synovial fluid, albumin can become cationic in an acid medium and will precipitate with anionic hyaluronan, especially if the salt concentration is low and is in a desiccated environment.13, 14 Thus, under the appropriate concentration of cationic components, commercial hyaluronan could precipitate out of the solution and deposit on various surfaces. Residual cationic detergents and proteins may eventually be present in the eye due to any reused device that has not been properly cleaned (not reported in the two cases described here), or due to conditions inherent to the eye itself. It has also been demonstrated that use of saline rather than sterile water in the cleaning and sterilizing process will contribute to a precipitation problem. The adhesive nature of the AcrySof® material would make removal of any precipitates from the lenses' surfaces more difficult.
General rules that help in the prevention of precipitation problems include thorough rinsing of any reused device (when applicable). Also, it is important to follow the manufacturer's recommendations regarding loading of IOLs and storage of any OVD. Special attention should be paid to minimize the period of contact between the OVD and the lens before injection into the eye. Any deposits seen on the lens or in the OVD solution should warrant immediate explantation of the lens if the material is not easily removed. In addition, a culture of the lens, OVD solution, and cartridges should be performed. Finally, a thorough review of all cleaning/sterilization techniques should be undertaken.
References
Davison JA . Clinical performance of Alcon SA30AL and SA60AT single-piece acrylic intraocular lenses. J Cataract Refract Surg 2002; 28: 1112–1123.
Jensen MK, Crandall AS, Mamalis N, Olson RJ . Crystallization on intraocular lens surfaces associated with the use of Healon GV. Arch Ophthalmol 1994; 112: 1037–1042.
Olson RJ, Caldwell KD, Crandall AS, Jensen MK, Huang SC . Intraoperative crystallization on the intraocular lens surface. Am J Ophthalmol 1998; 126: 177–184.
Nevyas AS, Raber IM, Eagle Jr RC, Wallace IB, Nevyas HJ . Acute band keratopathy following intracameral Viscoat. Arch Ophthalmol 1987; 105: 958–964.
Package insert for Healon GV. Physicians' Desk Reference, 48th ed. Medical Economics Data Production Co: Montvale NJ, 1994.
Binder PS, Deg JK, Kohl FS . Calcific band keratopathy after intraocular chondroitin sulfate. Arch Ophthalmol 1987; 105: 1243–1247.
Brinton GS, Topping TM, Hyndiuk RA, Aaberg TM, Reeser FH, Abrams GW . Posttraumatic endophthalmitis. Arch Ophthalmol 1984; 102: 547–550.
Das T, Choudhury K, Sharma S, Jalali S, Nuthethi R . Endophthalmitis Research Group. Clinical profile and outcome in Bacillus endophthalmitis. Ophthalmology 2001; 108: 1819–1825.
Greenspon EA . A pathogenic Bacillus subtilis isolated from the eye. Am J Ophthalmol 1918; 1: 316–318.
Hemady R, Zaltas M, Paton B, Foster CS, Baker AS . Bacillus-induced endophthalmitis: new series of 10 cases and review of the literature. Br J Ophthalmol 1990; 74: 26–29 (Erratum in: Br J Ophthalmol 1991; 75:255).
Roy M, Chen JC, Miller M, Boyaner D, Kasner O, Edelstein E . Epidemic Bacillus endophthalmitis after cataract surgery I: acute presentation and outcome. Ophthalmology 1997; 104: 1768–1772.
Chen JC, Roy M . Epidemic Bacillus endophthalmitis after cataract surgery II: chronic and recurrent presentation and outcome. Ophthalmology 2000; 107: 1038–1041.
Schubert M . Intercellular macromolecules containing polysaccharides. Biophys J 1964; 71 (Suppl.): 119–138.
Chakrabarti B, Park JW . Glycosaminoglycans: structure and interaction. CRC Crit Rev Biochem 1980; 8: 225–313.
Acknowledgements
D Zhao, PhD (Electron Microscopy Center, University of South Carolina, Columbia, SC, USA) provided assistance with surface analyses, and CC Nelson, PhD, and P Krishna, PhD (University of Utah Mass Spectrometry Core Facility, Salt Lake City, UT, USA) provided assistance with protein analyses. James P Gilman, CRA, and Elizabeth Snodgrass, CRA (John A Moran Eye Center, University of Utah, Salt Lake City, UT, USA), assisted with the photo documentation. This work was supported by the Research to Prevent Blindness Olga Keith Weiss Scholar Award (to Liliana Werner, MD, PhD). The authors have no financial interest in any product mentioned in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at ASCRS Symposium on Cataract, IOL, and Refractive Surgery, May 2004, San Diego, CA, USA
Synopsis: Surface deposits found on two hydrophobic acrylic intraocular lenses immediately after implantation into the eye may have been caused by crystallization of ophthalmic viscosurgical devices used for the loading of the lenses into the cartridges
Rights and permissions
About this article
Cite this article
Hickman, M., Werner, L., Mamalis, N. et al. Intraoperative explantation of two single-piece hydrophobic acrylic intraocular lenses due to surface deposits. Eye 20, 1054–1060 (2006). https://doi.org/10.1038/sj.eye.6702124
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702124