Abstract
Aims
To evaluate the efficacy and safety of low-dose (2 mg in 0.05 ml) intraocular triamcinolone injection for patients with uveitis-related cystoid macular oedema and/or intractable intraocular inflammation.
Patients and methods
Retrospective clinical case series.
Results
Cystoid macular oedema was eliminated in 24/30 eyes (80%). Intractable intraocular inflammation was eliminated in 4/8 eyes (50%). Snellen visual acuity was improved by two lines or more after 14/36 injections (38.9%). Intraocular pressure rose to above 21 mmHg after 8/36 injections (22%). There were no major complications.
Conclusions
Low-dose (2 mg in 0.05 ml) intraocular triamcinolone acetonide injection is safe and effective for the management of refractory uveitic macular oedema. Its usefulness in controlling inflammation alone is questionable.
Similar content being viewed by others
Introduction
Previous reports1, 2, 3 have shown the efficacy of intraocular triamcinolone injection in the management of small numbers of patients with inflammatory macular oedema (CMO) and have described the complication profile. We wished to evaluate further the efficacy and safety of low-dose pars plana intravitreal injection of triamcinolone acetonide for the treatment of intractable uveitis or inflammatory CMO within the context of a specialist uveitis service.
Patients and methods
All patients (Table 1) were attending the Manchester Uveitis Clinic under the care of one of us (NPJ). All had inflammatory CMO (as judged by slit-lamp biomicroscopy) and/or intractable uveitis responding inadequately to oral steroid and/or oral immunosuppression. The technique was not used in those with a history of raised intraocular pressure (IOP) after steroid treatment. All patients were given an information pamphlet describing the technique, its likely temporary effect, and describing possible complications including infection, inflammation, raised IOP, cataract, retinal detachment, and the possible need for surgery to treat a complication. Informed consent was given.
All procedures took place in an ophthalmic operating theatre. Almost all injections were given using local anaesthesia. Topical anaesthetic was instilled, followed by Povidone–Iodine conjunctival irrigation. Two perilimbal subconjunctival blebs of lignocaine 2% were then injected at points for fixation and injection. Ocular pressure was applied for 10 min.
Triamcinolone acetonide (Kenalog®, Bristol-Myers Squibb, Middlesex UK) was thoroughly shaken and drawn into a 1 ml syringe. Suspension was expelled until 0.1 ml remained. A 27-gauge or 30-gauge cannula was mounted and further suspension was ejected until 0.05 ml (2 mg) remained. The injection was made immediately to avoid drug precipitation within the syringe.
The eye was exposed with a Clarke speculum and fixated with Hoskins forceps, the opposite sclera was marked 3.5 mm behind the limbus, and the injection was given towards the centre of the globe. IOP was assessed digitally and optic disc perfusion examined if in doubt. Anterior chamber (AC) paracentesis was performed if necessary. Topical chloramphenicol was applied for 5 days postoperatively.
Patients were warned of probable minor postoperative discomfort, minor subconjunctival haemorrhage, and a cloud in the vision for 2–3 days. A first-day postoperative review was abandoned as unnecessary after a few patients, review 2 weeks after treatment, then 4–6 weeks later, becoming standard.
Snellen visual acuity and IOP were assessed at each visit, as was anterior and posterior segment inflammation and CMO, assessed using slit-lamp biomicroscopy. Preoperative IOP (mean of measurements within 2 months of surgery) was compared with postoperative IOP (highest of measurements within two months after surgery). Patients were followed for at least 2 months postoperatively.
Results
In total, 36 injections were performed on 33 eyes of 29 patients (24 female, five male), four undergoing bilateral injections and three, repeat injections. The mean age at treatment was 51.4 years (13–72 years). Before treatment, intractable CMO was present in 30 eyes and intractable inflammation (panuveitis or vitritis) in eight eyes. The mean follow-up was 13.5 months (2–26 months).
There was one peroperative complication; a 30-gauge cannula was blocked by triamcinolone, and the cannula blew off the syringe on attempted injection. No intraocular damage was caused and the injection was repeated. An AC paracentesis was necessary after injection in only one eye. There were no cases of endophthalmitis, cataract, vitreous haemorrhage, or retinal detachment.
Complete clinical resolution of CMO was observed in 24/30 eyes (80%) and partial resolution in a further three. There was no clinical change in three eyes. AC activity was detected in 17 eyes preoperatively but in only four eyes postoperatively. In one eye, a small postoperative pseudohypopyon resolved rapidly and in another, mild AC activity was detected in a previously quiet eye. Vitreous activity, detected in 18 eyes preoperatively, resolved completely in 13 eyes (72.2%) and improved in a further four. On one occasion there was mild vitreous activity in a previously quiet eye. Of the eight eyes with intractable intraocular inflammation, this was eliminated in four and reduced in one (improved in 5/8, 62.5%). In two eyes an epiretinal membrane was detected de novo, 2 months after injection.
A postoperative rise in IOP of 5 mmHg or more was measured in 14 eyes (38.8%), and of 10 mmHg or more in seven eyes (19.4%), but a rise to >21 mmHg (maximum 36 mmHg) was noted in only eight eyes (22.2%). Two eyes with IOP >30 mmHg received temporary topical antiglaucoma medication and none required surgical intervention.
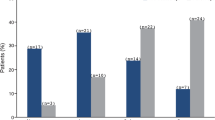
Snellen visual acuity (Figure 1) improved by two lines or more after 14 injections (38.9%) and by one line or more in 22 eyes (61.1%). There was no change in 12 eyes (33.3%) and a deterioration in two eyes (both 6/24 to 6/60 with intractable CMO). Of the eyes with a preinjection acuity of 6/60 or better, 12/24 (50%) showed a two-line improvement or more. Of the eyes worse than 6/60, only 2/12 (16.6%) showed a two-line improvement or more, but six showed a one-line improvement or better (in all of which, CMO was eliminated). Of those 14 eyes which failed to show an improvement in visual acuity following treatment, 10 had dry but scarred maculae, and four maculae remained oedematous.
Discussion
Persistent CMO and immunosuppression-resistant inflammation are regularly encountered in patients attending a uveitis clinic. These problems are sight threatening and their management problematic. Inflammatory CMO has been treated with topical and oral NSAIDs, oral acetazolamide, oral steroid and immunosuppressive, periocular depot steroid injection, and laser treatment. All methods are useful in some, but none is reliable for all, and optimal management is controversial.
Intravitreal triamcinolone injection was first used in an animal model of proliferative vitreoretinopathy in 1980,4 following which the tolerability and pharmacodynamics were observed.5, 6 The technique has now been used in a large number of patients for a variety of indications including idiopathic, postcataract, inflammatory, diabetic, postvenous occlusion, and other forms of CMO. Dosages have varied from 21 to 25 mg.7 Using a 4 mg injection in nonvitrectomised eyes, the mean elimination half-life was found to be 18.6 days,8 but with significant variation in peak concentrations. Using this dose, detectable triamcinolone could be present for 3 months. However, using a 25 mg dose, Jonas9 found that triamcinolone could be detected up to 18 months from injection.
Complications of intravitreal triamcinolone injection include raised IOP in a substantial proportion of patients10, 11, 12 (the need for trabeculectomy being reported1). Infectious and noninfectious endophthalmitis7, 13, 14 are both well described, Moshfegi et al13 finding as many as eight in 922 injections (0.87%) in a multicentre retrospective study, resulting in three blind eyes and one enucleation. There may be progression of cataract.12 It is suggested14 that formulation constituents, possibly benzyl alcohol 0.99%, may provoke sterile inflammation in a small number of patients. After removing additives by microfiltration,7 no cases of sterile endophthalmitis were encountered in 454 procedures. It is encouraging that a prospective observer-blinded randomised study, while demonstrating the frequency of IOP rise in the treated group, showed no sight-threatening events in a cohort of 75 eyes observed over 3 years from injection.12
The technique has been reported in small groups of patients with uveitis2 or uveitic CMO1, 3 with generally favourable results, but with some concerns about post-operative IOP. It has also been used with some success to treat serous retinal detachment in the Vogt–Koyanagi–Harada syndrome15 and anecdotally in sympathetic uveitis.16 However, this is to our knowledge the largest series of patients with uveitis yet reported. We chose to use the low dose of 2 mg of triamcinolone in 0.05 ml, which has previously been found to be effective in a small group of patients with uveitic macular oedema,1 which reduces injection volume and which logically should reduce the incidence of induced glaucoma. This and the use of preinjection ocular pressure ensured that only one paracentesis was required in 36 procedures.
Triamcinolone is capable of rapid precipitation, which may block a 30-gauge cannula. On one occasion this caused the cannula to be ‘fired’ into the eye. We now use only Leuer-lock syringes for this technique. It is recommended that triamcinolone is only drawn up immediately prior to injection, which must be made with care; the alternative 27-gauge cannula is in our experience felt more often by the patient despite subconjunctival anaesthesia.
We have been impressed by the efficacy of this low-dose triamcinolone injection, which has eliminated clinically visible CMO in 80% of eyes, and improved Snellen acuity by two lines or more in nearly 40%. In general, these are patients in whom traditional treatment methods have already failed. Visual improvement is closely correlated with the removal of CMO; although the technique has been effective in removing intraocular inflammation in some eyes, this alone has not been reflected in an improvement in VA and its usefulness is questionable.
Using this low dose of 2 mg, few eyes developed a problematic IOP rise and with the exception of one evanescent pseudohypopyon, no episodes of significant inflammation were encountered. This technique can achieve control of an otherwise difficult situation in selected cases. However, those with a very poor pre-injection VA fare much less well.
On four occasions we injected an eye twice with triamcinolone; in all four cases there had been elimination or reduction in CMO after the first injection. The second injection, given after an interval of 6 months or longer, failed in two eyes and was successful in two eyes (eliminating CMO in both and increasing visual acuity). The safety of repeated injection is unproven; we have not yet given three injections to an eye. Neither have we yet explored the possibility of higher-dose second injection for those eyes where CMO was unaffected by a single 2 mg injection.
Using a 4 mg injection, an IOP rise of 10 mmHg or more was seen in 27.9% of eyes.10 Using 2 mg our rate was 19.4%. Using a 25 mg injection, 52% of eyes developed an IOP rise to 21 mmHg or more.17 Using 2 mg our rate was 22.2%. We are confident that the use of a lower dose has reduced the incidence and severity of postoperative IOP rise in comparison with other studies.
In conclusion, intravitreal injection of low-dose triamcinolone acetonide (2 mg in 0.05 ml) is effective in the removal of inflammatory macular oedema and may enhance vision even when oedema has been longstanding. It is often effective in the reduction of intraocular inflammation. It is associated with a small but well-recognised incidence of complications, some of which may cause blindness. Using this low dosage, these risks are reduced. We now consider intraocular triamcinolone injection in the following circumstances for patients with uveitis:
-
1
To treat inflammatory macular oedema unresponsive to oral steroid or immunosuppression, or after failure of orbital floor depot steroid injection.
-
2
To ascertain visual potential (in order to plan future treatment) for patients with longstanding uveitis including scarred, oedematous maculae.
-
3
To treat patients with unstable uveitis and macular oedema, prior to planned cataract surgery.
References
Antcliff RJ, Spalton DJ, Stanford MR, Graham EM, ffytche TJ, Marshall J . Intravitreal triamcinolone for uveitis cystoid macular edema: an optical coherence tomography study. Ophthalmology 2001; 108: 765–772.
Sanchez JM, Sanchez J . Intravitreal injection of triamcinolone acetonide in non infectious uveitis. Arch Soc Esp Oftalmol 2001; 76: 661–664.
Young S, Larkin G, Branley M, Lightman S . Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Exp Ophthalmol 2001; 29: 2–6.
Tano Y, Chandler D, Machemer R . Treatment of intraocular proliferation with intravitreal injection of triamcinolone acetonide. Am J Ophthalmol 1980; 90: 810–816.
McCuen BW, Bessler M, Tano Y, Chandler D, Machemer R . The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol 1981; 91: 785–788.
Schindler RH, Chandler D, Thresher R, Machemer R . The clearance of intravitreal triamcinolone acetonide. Am J Ophthalmol 1982; 93: 415–417.
Jonas JB, Kreissig I, Degenring RF . Endophthalmitis after intravitreal injection of triamcinolone acetonide. Arch Ophthalmol 2003; 121: 1663–1664.
Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB, Miller M . Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology 2003; 110: 681–686.
Jonas JB . Intraocular availability of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol 2004; 137: 560–562.
Bakri SJ, Beer PM . The effect of intravitreal triamcinolone acetionide on intraocular pressure. Ophthalmic Surg Lasers Imaging 2003; 34: 386–390.
Wingate RJ, Beaumont PE . Intravitreal triamcinolone and elevated intraocular pressure. Aust NZ J Ophthalmol 1999; 27: 431–432.
Gillies MC, Simpson JM, Billson FA, Luo W, Penfold P, Chua W et al. Safety of an intravitreal injection of triamcinolone: results from a randomised clinical trial. Arch Ophthalmol 2004; 122: 336–340.
Moshfegi DM, Kaiser PK, Scott IU, Sears JE, Benz M, Sinisterra JP et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol 2003; 136: 791–796.
Nelson ML, Tennant MTS, Sivalingam A, Regillo CD, Belmont JB, Martidis A . Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina 2003; 23: 686–691.
Andrade RE, Muccioli C, Farah ME, Nussenblatt RB, Belfort R . Intravitreal triamcinolone in the treatment of serous retinal detachment in Vogt–Koyanagi–Harada syndrome. Am J Ophthalmol 2004; 137: 572–574.
Jonas JB . Intravitreal triamcinolone acetonide for treatment of sympathetic ophthalmia. Am J Ophthalmol 2004; 137: 367–368.
Jonas JB, Kreissig I, Degenring R . Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol 2003; 87: 24–27.
Acknowledgements
We thank Dr A Armoogum for assistance with data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no financial or other interest in any proprietary product or medication discussed in this article
Rights and permissions
About this article
Cite this article
Das-Bhaumik, R., Jones, N. Low-dose intraocular triamcinolone injection for intractable macular oedema and inflammation in patients with uveitis. Eye 20, 934–937 (2006). https://doi.org/10.1038/sj.eye.6702063
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702063
Keywords
This article is cited by
-
Photodynamic therapy combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization
Frontiers of Medicine in China (2007)