Abstract
Purpose
To investigate whether inflammatory responses are more severe in uveitic eyes than nonuveitic eyes when acrylic intraocular lens (IOL) is implanted after cataract surgery.
Methods
Clear lens removal (phacoemulsification and aspiration) was conducted and the hydrophobic acrylic IOL (AR40e, AMO) was implanted in adult albino rabbits. Just after the operation, rabbits were divided into two groups. One group (nine rabbits) received intravitreal injection of lipopolysaccharide (LPS, 200 ng/10 μl) into both eyes to induce endotoxin-induced uveitis (EIU) and the other group (nine rabbits) received intravitreal injection of phosphate-buffered saline (PBS, 10 μl) into both eyes as the control. Aqueous humour (AH) and IOLs were harvested 1, 3 , and 7 days after the intravitreal injection. The infiltrating cell number in AH was counted and the protein concentration of AH was measured. IOLs were evaluated morphologically.
Results
At 1 day after intravitreal injection, both the infiltrating cell number in AH and protein concentration of AH were significantly higher in the LPS-injected group than in the PBS-injected group. Similarly, more inflammatory cells attached to the surfaces of the IOLs in the LPS-injected group. However, 7 days later, inflammatory reactions subsided and no clear differences in any of the parameters examined were observed between the two groups.
Conclusions
At 7 days after the operation, inflammatory reactions in eyes implanted with the hydrophobic acrylic IOLs were similar in uveitic eyes and nonuveitic eyes. The data suggest that the hydrophobic acrylic IOLs may be suitable for patients with uveitis.
Similar content being viewed by others
Introduction
Cataract, a common complication of uveitis, can cause visual disturbance.1 In such cases, cataract extraction followed by intraocular lens (IOL) implantation is necessary to improve visual acuity. Intraocular inflammation after cataract surgery is well known to be more common and severe in patients with uveitis than in patients without ocular complications.2 Recent advances in cataract surgery, including use of phacoemulsification machines and newly developed IOLs, have suppressed ocular inflammation after cataract surgery in patients with uveitis. However, some uveitic patients still develop severe intraocular inflammation after cataract surgery.3
In vitro studies conducted with uveitogenic T cells or splenocytes demonstrated that uveitogenic cells attached to the surfaces of IOLs more than control cells obtained from naïve rats.4 In addition, the attachment to the surfaces of IOLs by uveitogenic cells was further augmented by the presence of uveitogenic antigen.5 These results support the clinical observation that a large number of cells attach to the surfaces of IOL in patients with uveitis.
Few in vivo studies in animal models of uveitis are available. Lundgren et al6 examined cellular reactions after IOL (polymethyl methacrylate (PMMA) lens and heparin-surface modified (HSM) lens) implantation in rabbits with endotoxin-induced uveitis (EIU), a model of acute anterior uveitis. Small incision cataract surgery has recently become popular and therefore, foldable acrylic IOLs are increasingly being used. Indeed, in 2002, a survey by the American Society of Cataract and Refractive Surgery demonstrated that acrylic IOLs were preferred by 69% of cataract surgeons.7 The biocompatibility of acrylic IOLs in uveitic patients has been examined;8, 9, 10 however, comparison of implantation of acrylic IOLs in acute uveitic eyes with nonuveitic eyes has not yet been reported. Here, we investigated the influence of acute uveitis on the ocular inflammatory reaction following implantation with acrylic IOL.
Materials and methods
Animals
Adult albino rabbits (New Zealand white, 2–2.5 kg) were maintained individually under conventional conditions at the Kochi Medical School animal facility. All research adhered to the guidelines of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
IOL
The lens used in this study was a three-piece acrylic lens (AR40e, AMO, Santa Anna, CA, USA).
Operation procedure
The rabbits were anaesthetized with ketamine hydrochloride and xylazine chloride (35 and 5 mg/kg body weight i.m., respectively). Pupil dilatation was performed by instillation of cyclopentolate hydrochloride three times. Local anaesthesia was induced by instillation of oxybuprocaine hydrochloride three times concomitantly with cyclopentolate hydrochloride. All the rabbits underwent a standardized small-incision phacoemulsification with capsular bag IOL implantation using an injector (The Unfolder Emerald, AMO, Santa Anna, CA, USA). All the rabbits underwent bilateral phacoemulsification with IOL implantation. Surgery was uneventful. All the operations were performed by the same surgeon (YK). Capsulorrhexis was conduced after a 3.2 mm near-clear corneal tunnel was made. Then, a phacofracture in a capsular bag and automated irrigation aspiration of the cortical remnants were conducted. The IOL was implanted in the capsular bag. Finally, suture with a single 10-0 nylon stitch followed. Just after these procedures were performed, rabbits were divided into two groups. One group (nine rabbits) received intravitreal injection of phosphate-buffered saline (PBS, 10 μl) into both eyes and the other group (nine rabbits) received intravitreal injection of Salmonella typhimurium lipopolysaccharide (LPS, 200 ng/10 μl: Difco Laboratories, Detroit, MI,USA) into both eyes. Then, all the rabbits received eye drops of 0.5% levofloxacin and 0.1% betamethasone in both eyes. Thereafter, the rabbits were not treated with any eye drops. Three rabbits (six eyes) in each group were killed for evaluation 1, 3, and 7 days after the operation.
Clinical evaluation
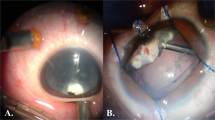
At the time of killing, photos of the anterior segments of the eyes were taken to compare clinical inflammatory severity between the two groups. Conjunctival hyperaemia, cloudiness of the anterior chamber (AC), and fibrin formation in AC were evaluated.
Evaluation of aqueous humour
The infiltrating cell number in the aqueous humour(AH) was counted by Trypanblue exclusion. After cell counting, aqueous humour was centrifuged and supernatants were subjected to measurement of protein quantification using the Bicinchoninic Acid (BCA) Protein Assay Kit (PIERCE, Rockford, IL,USA).
Evaluation of IOL
Extracted IOLs were fixed in 2.5% glutaraldehyde. Photos of fixed IOLs were taken under biomicroscope (MZ16FA, Leica Microsystems, Wetzlar, Germany) and cells attached to the surfaces of IOLs were counted. Counting was performed in a masked fashion. Five different portions of IOLs (each area was 0.01 mm2) were drawn for counting, as shown in Figure 1. The average±SD of the five different areas was presented as data.
Statistical analysis
Comparisons of cell numbers and protein concentrations in the AH samples and the cell numbers attached to the surface of IOLs were performed by Student's t-test. P-values of <0.05 were considered statistically significant.
Results
Clinical evaluation
As shown in Figure 2, conjunctival hyperaemia was more prominent in the LPS-injected group at all the examined time points. Similarly, AC cloudiness was more pronounced in the LPS-injected group. AC cloudiness was most prominent 24 h after the operation in both groups and gradually improved. Fibrin formation in AC was greater in the LPS-injected group up to 3 days after the operation; however, on day 7, fibrin formation in AC did not differ between the groups.
Inflammatory reaction in the AC
To confirm that intravitreal injection of LPS induced EIU, the infiltrating cell numbers and protein concentrations in AH were examined. At 1 day after the operation, significantly more cells infiltrated into AH in the LPS-injected group (4.9 × 106 cells/ml) than in the PBS-injected group (7.5x105 cells/ml; Figure 3). The infiltrating cell number gradually declined after the operation (Figure 3); the infiltrating cell number was significantly higher 3 days after the operation, but was not significantly different from the control group on day 7 (Figure 3). The protein concentration in AH was significantly higher in the LPS-injected group than in the PBS-injected group on both days 1 and 3 (Figure 4). However, on day 7, the protein concentration did not differ significantly (Figure 4). Interestingly, the protein concentration in the AH of the LPS-injected group was higher on day 3 than on day 1 (Figure 4). These data confirmed that intravitreal injection of LPS induced EIU in rabbits.
Deposition of inflammatory cells on the surface of IOL
To evaluate EIU effects on implanted IOLs, we extracted IOLs and processed them for morphological analysis. At 1 day after the operation, significantly more cells attached to the surface of IOLs in the LPS-injected group than in the PBS-injected group (Figures 5 and 6) and the difference persisted up to 3 days but not 7 days after the operation (Figures 5 and 6). Higher magnification microscopy demonstrated that most of the attaching cells were round on day 1, while spindle-shaped cells were dominant on days 3 and 7 (data not shown).
Discussion
Here, we demonstrate that inflammatory cell attachment to the surfaces of the hydrophobic acrylic IOLs is similar between uveitic and nonuveitic eyes 7 days after cataract surgery. To date, the biocompatibility of acrylic IOL implantation in uveitis eyes has not been compared to implantation in nonuveitic eyes under the same controlled environment. Thus, the data presented here will help evaluate whether or not the hydrophobic acrylic IOL is suitable for use in patients with uveitis.
Significantly more inflammation and cell attachment to the surfaces of IOLs were noted in the LPS-injected group at the earlier time points examined on days 1 and 3. However, on day 7, the augmented inflammatory reaction in the LPS-injected group declined to the not significantly different level of the PBS-injected group, although the reaction was slightly more severe. This finding suggests that after the inflammatory peak fades, cells attached to the surface of the IOL become dislodged from the acrylic IOL. The composition of the IOL used, AR40e, may cause inflammatory cells to dislodge because of its hydrophobicity.11 Notably, anti-inflammatory drugs such as steroids were only administered just after the operation. Therefore, these data can be considered representative of the natural course of inflammation induced by the operation and intravitreal injection.
Comparison of the biocompatibility of uveitis with acrylic IOLs and IOLs composed of other types of biomaterials is clearly necessary. Nevertheless, together with the increasingly widespread use of acrylic IOL,7 this study suggests that the hydrophobic acrylic IOLs can be implanted in patients with uveitis. Phenotypic analyses of cells attaching to the surfaces of IOLs are now under investigation and future studies will be focused on the comparison with other types of IOLs composed of different biomaterials.
References
Rosenberg KD, Feuer WJ, Davis JL . Ocular complications of pediatric uveitis. Ophthalmology 2004; 111: 2299–2306.
Rojas B, Zafirakis P, Foster CS . Cataract surgery in patients with uveitis. Curr Opin Ophthalmol 1997; 8: 6–12.
McColgin AZ, Heier JS . Control of intraocular inflammation associated with cataract surgery. Curr Opin Ophthalmol 2000; 11: 3–6.
Yamakawa N, Tanaka T, Shigeta M, Hamano M, Usui M . Surface roughness of intraocular lenses and inflammatory cell adhesion to lens surfaces. J Cataract Refract Surg 2003; 29: 367–370.
Tanaka T, Yamakawa N, Mizusawa T, Usui M . Interaction between inflammatory cells and heparin-surface-modified intraocular lens. J Cataract Refract Surg 2000; 26: 1409–1412.
Lundgren B, Holst A, Tarnholm A, Rolfsen W . Cellular reaction following cataract surgery with implantation of the heparin-surface-modified intraocular lens in rabbits with experimental uveitis. J Cataract Refract Surg 1992; 18: 602–606.
Leaming DV . Practice styles and preferences of ASCRS members—2003 survey. J Cataract Refract Surg 2004; 30: 892–900.
Abela-Formanek C, Amon M, Schauersberger J, Schild G, Kolodjaschna J, Barisani-Asenbauer T et al. Uveal and capsular biocompatibility of 2 foldable acrylic intraocular lenses in patients with uveitis or pseudoexfoliation syndrome: comparison to a control group. J Cataract Refract Surg 2002; 28: 1160–1172.
Abela-Formanek C, Amon M, Schild G, Schauersberger J, Kolodjaschna J, Barisani-Asenbaum T et al. Inflammation after implantation of hydrophilic acrylic, hydrophobic acrylic, or silicone intraocular lenses in eyes with cataract and uveitis: comparison to a control group. J Cataract Refract Surg 2002; 28: 1153–1159.
Abela-Formanek C, Amon M, Schauersberger J, Kruger A, Nepp J, Schild G . Results of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in uveitic eyes with cataract: comparison to a control group. J Cataract Refract Surg 2002; 28: 1141–1152.
Sacu S, Menapace R, Buehl W, Rainer G, Findl O . Effect of intraocular lens optic edge design and material on fibrotic capsule opacification and capsulorhexis contraction. J Cataract Refract Surg 2004; 30: 1875–1882.
Acknowledgements
We appreciate the excellent help provided by Ms Kazuyo Fukata, Mr Kazutaka Hirabayashi, Dr Satoko Sugimoto, Dr Takashi Nishiuchi, and Dr Junya Takami.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koura, Y., Fukushima, A., Nishino, K. et al. Inflammatory reaction following cataract surgery and implantation of acrylic intraocular lens in rabbits with endotoxin-induced uveitis. Eye 20, 606–610 (2006). https://doi.org/10.1038/sj.eye.6701975
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701975
Keywords
This article is cited by
-
In Vitro, In Vivo, and In Silico Evaluation of the Bioresponsive Behavior of an Intelligent Intraocular Implant
Pharmaceutical Research (2014)