Abstract
Objective
To report the clinical outcome of a patient who received high-dose intravitreal triamcinolone acetonide as treatment for severe macular oedema secondary to adult Coat's syndrome.
Method
Case report.
Results
A 74-year-old Indian man complaining of chronic gradual blurring of vision in the left eye was found to have adult Coat's syndrome with severe macular oedema. He received 25 mg of intravitreal triamcinolone acetonide following unsuccessful resolution with grid laser. Optical coherence tomography (OCT) demonstrated up to 75% decrease in macular oedema that was evident even after 9 months follow-up. However, there was no significant improvement in visual acuity.
Conclusion
Intravitreal triamcinolone is a reasonable option in reducing severe macular oedema in cases of adult Coat's syndrome.
Similar content being viewed by others
Introduction
Coat's syndrome is primarily a childhood condition but is known to affect the adult population less commonly. The disease is characterized by telangiectactic and aneurysmal retinal vessel dilatations with massive exudation. Adult onset disease is generally reported to be less severe and visual loss is usually secondary to excessive exudation from abnormal vessels causing cystoid macular oedema and circinate lipid exudations.1 Management is controversial. Although focal and grid laser have been routinely used and has been reported to reduce severe visual loss in some cases of severe macular oedema, most cases remain refractory to treatment.2, 3
This case reports the clinical findings and outcome of a patient with severe macular oedema in adult Coat's syndrome treated with high-dose intravitreal triamcinolone.
Patient and method
A 74-year-old Indian man presented with chronic, gradual blurring of vision in his left eye. The visual acuity was counting fingers closely. Anterior segment examination showed a pseudophakic eye with deep anterior chamber without iris neovascularization. Ophthalmoscopy showed retinal telangiectasias with haemorrhages and diffuse hard exudates associated with pronounced thickening of the macula (Figure 1). Fluorescein angiography showed massive leakage in the foveal area with areas of capillary fallout. Ocular coherence tomography (OCT) showed a very thickened macula (919 μm) (Figure 2a). Two sessions of grid laser using the frequency-doubled YAG laser (532 nm, Iris Medical) were performed. The right eye was normal on examination and fluroescein angiography with a visual acuity of 20/20.
At 3 months postlaser, there was no improvement noted in the amount of macular oedema on OCT (843 μm) and visual acuity. The patient was offered treatment with intravitreal triamcinolone acetonide (TAA) (Kenalog®).
Under topical anaesthesia, 25 mg of TAA suspension in 0.05 ml Ringer's solution was injected trans-pars plana using a gauge 30 needle in the inferotemporal quadrant. This was followed by assessment of the central retinal artery perfusion using indirect ophthalmoscopy. The TAA suspension was prepared by drawing 0.6 ml of TAA suspension into a tuberculin syringe of which 0.4 ml was discarded and replaced with Ringer's lactate solution. The new suspension was then allowed to settle for 30 min to separate the crystals from the vehicle. Passing through a 0.2 μm Millipore filter, the supernatant containing the vehicle was removed and the remaining crystals were resuspended with Ringer's lactate solution drawing to 1 ml mark. This step was repeated three times. After the last washing, the crystals were resuspended in 0.05 ml of Ringer's lactate solution. This was used for the injection. The patient was reviewed on the first postinjection day, at 1 week, 1 month, 3 months, and at 3 monthly intervals subsequently.
Results
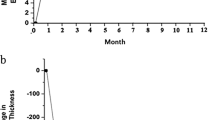
OCT on the first week postinjection showed that central foveal thickness decreased from 919 to 538 μm (41. 5%). This reduced to 281 μm at 1 month (69.8% from baseline), 182 μm at 3 months (80.5%), 233 μm at 6 months follow-up (74.7%), and 225 μm at 9 months follow-up (75.8%) (Figure 2b). This correlated with a clinical improvement in macular oedema visualized on slit-lamp biomicroscopy and serial fundus photographs. Visual acuity unfortunately did not improve at any point of follow-up and remained at counting fingers closely at 9 months postinjection.
The patient complained of acute floaters immediately after injection due to triamcinolone crystals in the mid-vitreous cavity. At 1 day postinjection the crystal were seen to have partially settled inferiorly and had resolved completely by the first month postinjection. Anterior chamber was quiet throughout the follow-up period. There were no other complications.
Discussion
This is the first report of successful resolution of severe macular oedema in adult Coat's syndrome with high-dose (25 mg) intravitreal triamcinolone. Several treatment modalities have been proposed for the management of Coat's disease including hormones, antibiotics, radiation, trans-scleral diathermy, and cryotherapy with minimal success.
Recent studies have shown triamcinolone to have promising effects in conditions with microangiopathy. It is believed that steroids can stabilize microcirculation and oedema. Coat's syndrome is also believed to be a chronic microangiopathy. In our case, intravitreal injection of triamcinolone acetonide effected a significant decrease in the macular oedema when conventional grid laser failed to do so. Significantly, the decrease in oedema persisted even 9 months after injection.
Triamcinolone has been widely investigated for its anti-inflammatory and angiostatic effect especially in severe diabetic maculopathy,1 macular oedema from vein occlusions,2 cystoid macular oedema in chronic uveitis3 and postcataract surgery,4 and exudative age-related macular degeneration.5 However, Jonas et al6 reported treating two patients with Coats’-like disease that did not respond to intravitreal triamcinolone. Unlike the reported cases, the exudation responded well to treatment in our patient. We feel that this could possibly reflect the status of the underlying retinal pigment epithelium and its ability to maintain a ‘dry’ internal milieu after adjunctive ‘antiproliferative’ therapy with intravitreal steroids.
Functionally, our patient did not show improvement in visual acuity in spite of anatomical regression of macular thickness. This was likely to be due to the chronic state of the macular oedema that had permanently damaged or altered the function of the photoreceptors before initiation of treatment. Moreover, it is known that once lipid plaques have deposited on the macula, it is usually irreversible and often lead to permanent visual loss. However, Khairallah et al7 demonstrated resolution of early hard exudates in severe diabetic maculopathy treated by primary intravitreal TAA.
Intravitreal triamcinolone is a reasonable adjunctive treatment in severe refractory macular oedema and in reducing subretinal fluid prior to conventional laser or cryotherapy of peripheral telangiectasia in adult Coat's syndrome. Early treatment to control postlaser ablation macular oedema and before macular lipid deposition with irreversible damage to retinal pigment epithelium may be beneficial in stabilizing visual outcomes.
References
Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular oedema: preliminary results of a prospective controlled trial. Ophthalmology 2004; 111: 218–224.
Jonas J, Kreissig I . Intravitreal triamcinolone acetonide as treatment of macular oedema in central retinal vein occlusion. Graefe's Arch Clin Exp Ophthalmol 2002; 240: 782–783.
Degenring R, Jonas J . Intravitreal injection of triamcinolone acetonide as treatment for chronic uveitis. Br J Ophthalmol 2003; 87: 361.
Jonas J, Kreissig I, Degenring R . Intravitreal triamcinolone acetonide for pseudophakic cystoid macular oedema. Am J Ophthalmol 2003; 136: 384–386.
Jonas JB, Akkoyun I, Budde WM, Kreissig I, Degenring RF . Intravitreal reinjection of triamcinolone for exudative age-related macular degeneration. Arch Ophthalmol 2004; 122: 218–222.
Jonas J . Intravitreal triamcinolone acetonide as treatment for extensive exudative retinal detachment. Br J Ophthalmol 2004; 88: 587–588.
Khairallah M, Yahia SB, Ladjimi A, Attia S, Megdiche Z, Zaouali S et al. Intravitreal triamcinolone acetonide for diabetic macular oedema with severe hard exudates. Program and abstracts of the American Academy of Ophthalmology 2003 Annual Meeting; Nov 15–18, 2003; Anaheim, CA PO117 (unpublished).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors declare that they have no financial or proprietary interest in the products mentioned or described in this paper.
Rights and permissions
About this article
Cite this article
Jarin, R., Teoh, S. & Lim, T. Resolution of severe macular oedema in adult Coat's syndrome with high-dose intravitreal triamcinolone acetonide. Eye 20, 163–165 (2006). https://doi.org/10.1038/sj.eye.6701828
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701828
Keywords
This article is cited by
-
Use of intravitreal triamcinolone and bevacizumab in Coats' disease with central macular edema
Graefe's Archive for Clinical and Experimental Ophthalmology (2011)
-
Role of intravitreal bevacizumab in adult onset Coats’ disease
International Ophthalmology (2011)