Abstract
Aim
To compare matrix metalloproteinase (MMP) localisation in anterior keratectomy (AK) and lamellar keratectomy (LK) wounds.
Methods
Wounds were produced in one eye of 24 rabbits. The AK wounds were made to approximately 120 μm in depth and then allowed to re-epithelialise. The LK wounds were of similar depth, but the anterior stroma and epithelium were replaced after a second deeper keratectomy had been performed. Immunohistochemistry was used to localise the MMP-1, -2, -3, and -9 at intervals from 4 h to 14 days following surgery. The contralateral eyes acted as controls.
Results
After an AK wound MMP-1 was present at the leading edge of migrating epithelium after 18 h, while MMP-2 and -9 were localised behind the advancing epithelial edge. The presence of these enzymes rapidly fell to low levels after epithelial closure. There was only faint MMP-3 localisation between days 3 and 7. After an LK wound, MMP-1, -3, and -9 were not detected in the stromal interface, but MMP-2 was present at all time points.
Conclusions
This study suggests that after an AK wound, MMP-1 is a key mediator of epithelial migration, while MMP-2 and -9, and to a lesser extent MMP-3, may participate in the remodelling of corneal stroma and the reformation of epithelial basement membrane. In contrast, an LK wound results in a much lower stimulus for MMP activation. The action of MMP-2 in stromal repair is thus partly independent of epithelial injury.
Similar content being viewed by others
Introduction
Matrix metalloproteinases (MMPs) are a group of zinc-dependent proteinases whose substrates include most components of the extracellular matrix and basement membrane. They are secreted in a latent form and are naturally regulated by tissue inhibitors of metalloproteinases (TIMPs). They play a central role in embryogenesis and development, and they also modulate wound healing by permitting cellular migration and subsequent wound contraction.1, 2, 3 In the normal cornea, only very low levels of MMP-2 are found as proenzyme.4, 5, 6 After injury, and in response to the release of cytokines, several MMPs in the cornea are upregulated by transcription or activation.7 Preformed MMP-9 (Gelatinase B) may also be released from the secretory granules of neutrophils recruited by any associated inflammation.8 It has been demonstrated that MMP-1 (interstitial collagenase), -2 (gelatinase A), -3 (stromelysin-1), and -9 participate in epithelial repair and stromal remodelling.5, 9 Their mechanism of action is uncertain, but MMP-1 is essential in vitro for the migration of corneal epithelial cells over a type I collagen matrix.10 It has also been noted that the subepithelial expression of MMP-9 parallels basement membrane degredation,11, 12 while MMP-2 and -3 produced by stromal fibroblasts may mediate long-term stromal remodelling and basement membrane synthesis.1, 4, 13
The development of laser refractive procedures such as laser in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK) has highlighted that the corneal response to injury depends on wound construction. The role of epithelial repair in the healing process appears to be critical. After lamellar keratectomy (LK) or LASIK, where the epithelium and basement membrane remain intact over the central cornea, there is less stromal response and fibrosis than if the anterior epithelium is removed.14, 15, 16 Wound healing after anterior keratectomy (AK) or PRK first requires epithelialisation over the exposed stromal substrate followed by keratocyte migration and activation, with the synthesis of new extracellular matrix,17, 18, 19 and the resulting scar tissue can cause a loss of corneal clarity and a regression of the refractive effect.18, 20, 21, 22 However, the advantage of a reduced stimulus for scarring following LASIK has to be balanced against the added risk of cutting a corneal flap. An understanding of the differences between the healing mechanism after PRK or LASIK may lead to techniques to promote epithelial closure after PRK while preventing keratocyte activation and scar formation. We have therefore studied the localisation of four MMPs following AK or LK in the rabbit eye.
Materials and methods
Animals received adequate care and humane treatment as stipulated by the ARVO statement for the use of animals in Ophthalmic and Vision research and in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986. A total of 24 pigmented Dutch belted rabbits weighing 1.0–1.5 kg were anaesthetised by intramuscular injection of ketamine 50 mg/kg (Willows Francis Veterinary, Crawley, Sussex, UK) and xylazine 3 mg/kg (Bayer, Bury St Edmunds, Suffolk, UK), plus subcutaneous buprenorphine 0.05 mg/kg (Reckitt & Coleman, Hull, North Humberside, UK) and a drop of topical proxymetacaine hydrochloride 0.5% (Chauvin) for postoperative analgesia.
Surgical procedure
In Group I (12 rabbits), a 120-μm incision was made in the central cornea with a 6-mm diameter Hessburg-Baron vacuum trephine (Altomed, Newcastle Upon Tyne, UK). An AK was then completed by sharp dissection. In Group II, a 120-μm AK was removed and kept in a moist chamber, while a second 120-μm depth keratectomy was made to remove mid-stromal tissue; the original anterior stroma and epithelium were then replaced in the correct orientation and sutured using eight interrupted 10/0 monofilament nylon sutures. Topical chloramphenicol 0.5% was given four times a day until the eye had re-epithelialised. Two animals were killed at each of the time points 4, 18, 24, 3, 7, and 14 days after surgery. The eyes were enucleated, with un-operated eyes acting as controls.
Histology and immunohistochemistry
Excised corneoscleral buttons were incubated at 37°C in 5 μ M Monensin (Sigma-Aldrich Company Ltd, Poole, Dorset, UK) for 6 h to retain intracellular MMP protein and prevent enzyme dispersal into the extracellular space.23 Incubation in the absence of monensin was not performed. Each cornea was then bisected and placed in OCT medium (Miles Elkhart, IN, USA) and stored at −70°C before 8 μm sections were cut with a cryostat. These were mounted on 3-aminopropyltriethoxysilane-coated glass slides,24 dried for 10 min, and placed in 4% paraformaldehyde for 30 min before washing in phosphate-buffered saline solution. Sections were permeabilised with 0.1% triton X-100 (Sigma) for 10 min, washed, and placed in 5% 4-chloronaphthol (Sigma) for 20 min. To reduce background staining, they were incubated with 5% rabbit serum (DAKO Ltd, High Wycombe, Bucks, UK) for 10 min at room temperature in a humid chamber. Excess serum was removed and 100 μl primary antibody (50 μg/ml sheep anti-rabbit MMP-1,25 sheep anti-human MMP-2,26 sheep anti-rabbit MMP-3,27 or sheep anti-pig MMP-928) in 5% normal rabbit serum,29 was added to sections and incubated at room temperature for 30 min in a humid chamber. These are all well-characterised antibodies with demonstrated activity against rabbit MMPs.26, 28 Normal sheep serum (50 μg/ml) was used as a negative control. Sections were washed, and then incubated with 100 μl of second antibody (rabbit F (ab′) 2 anti-sheep IgG (H+L)— fluoroisothiocynate (FITC) conjugated (Southern Biotechnology Associates Inc, Birmingham AL)) 1 : 100 in 5% rabbit serum for 30 min in a humid chamber and then washed. They were counterstained with 1% methyl green and mounted on slides using Citifluor. Slides were viewed by fluorescence microscopy (Leica DM, RBE) using a wideband FITC filter and photographed. Quantification of fluorescence was not performed.
Results
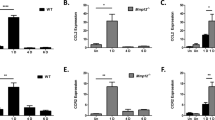
Re-epithelialisation following AK was typically complete by day 5 and no secondary breakdown was observed. Intracellular staining could not be reliably identified, and therefore we could not distinguish between the period of enzyme synthesis and residual binding of the enzyme to the extracellular matrix. MMP-1, -2, -3, or -9 were not detected in any of the uninjured control eyes at any time point. At 18 h following surgery, MMP-1 was present below the basal epithelial cells at the wound margin (Figure 1). The presence of MMP-1 persisted until day 7, but had diminished by day 14. MMP-2 and -9 were localised behind the leading edge of the basal epithelium (Figure 1), and were present until day 7, but staining had also markedly reduced at day 14. There was minor staining for MMP-3 in the anterior stroma at days 3 and 7 but not at other time points (data not shown).
Immunohistochemistry showing the presence of MMP -1, -2, -3, and -9, 18 h after wounding. Positive staining appears as green fluorescence (orange=autofluorescence) (A=anterior keratectomy, L=lamellar keratectomy. Arrows mark edge of AK wound, or interface of the lamellar keratectomy. *Leading edge of epithelium migrating right to left. Scale, line=100 μm).
After LK, the epithelium remained intact at all time points. The MMP-1, -3 and -9 were only detected at the margin of the LK wound where the epithelial surface had been breached. However, MMP-2 was detected at the intrastromal interface between 4 h and 7 days (Figure 1).
Discussion
We have compared the localisation of the MMP-1, -2, -3, and -9 following AK and LK in the rabbit eye. This model was chosen to reflect the healing responses following PRK or LASIK respectively. We observed the presence of MMP-1 adjacent to the basal epithelial cells at the margin of AK wounds in the region of most active cellular migration, and in the stroma beneath the wound surface. MMP -2 and -9 were predominantly localised behind the leading edge of migrating epithelium, which may indicate a role for these enzymes in stromal remodelling or early basement membrane assembly. MMP-2 was the only enzyme expressed in the stromal interface after LK suggesting that, unlike MMP -1 and -9, its participation in stromal remodelling may not depend on coexisting epithelial repair. These results correlate with the expression of MMP-1, -2, and -9 in dermal wounds.30, 31, 32 Although weak MMP-3 localisation was observed between days 3 and 7 in the anterior stroma after AK its function could not be further defined.
Other studies have reported MMP activity following corneal wounding.4, 5, 7, 9, 11, 12 Of relevance to this study, Azar et al33 used zymography to compare MMP expression after PRK or LASIK in the rabbit cornea and found that stromal levels of MMP-2 and -9 increased after both procedures, with high MMP-9 expression also localised in the epithelium during re-epithelialisation. The role of MMP-1 was not examined. Although the technique of zymography used in their study may provide a more sensitive index of enzyme activity, we feel that our use of immunofluorescence provides a better spatial localisation of enzyme activity in the wound. This potential role of MMP-9 as a mediator of stromal repair is highlighted by the observation that it can also activate TGF-β, which is a potent modulator of stromal scarring.34 The role of MMP-9 in corneal repair has been further studied in knockout mice, in which there is an increased rate of re-epithelialisation compared to wild types but with defects in basement membrane organization, supporting the concept that MMP-9 is essential for successful basement membrane formation.13 We demonstrated only transient and weak localisation of MMP-3 after AK but not LK, and this enzyme has previously been localised in newly synthesised stromal matrix at 1 week after laser AK in the rat cornea.35
In addition to the four MMPs we examined the role of other MMPs in corneal wound healing has been studied. MMP-7 (Matrilysin) is expressed in migrating basal epithelial cells after PRK,35 and studies in knockout mice suggest that MMP-7 may have a role in the inhibition of secondary neovascularisation of the wound.36 MMP-12, -13, and -14 expression has been studied in rat corneas following laser superficial keratectomy using reverse transcription-polymerase chain reaction, and the time course of expression of MMP-14 and -13 is similar to that of MMP-2 and –9, respectively, with a similar role in the regulation of stromal remodelling after wounding.37
In conclusion, our study supports the hypothesis that MMP -1, -2, and -9 are essential for healing of an anterior corneal wound in the rabbit. MMP-1 is probably fundamental to the process of corneal epithelial cell migration resulting in wound closure, whereas the presence of MMP-2 and -9, and to a lesser extent MMP-3, correlates with the period of keratocyte migration or activation at the wound site, basement membrane synthesis, and stromal remodelling. These findings suggest that development of specific MMP inhibitors may enable the wound healing processes to be dissected, permitting specific targeting of the stromal response while preserving epithelial migration.
References
Sivak JM, Fini ME . MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retinal Eye Res 2002; 21: 1–14.
Wong TTL, Sethi C, Daniels JT, Limb GA, Murphy G, Khaw PT . Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol 2002; 47: 239–256.
Daniels JT, Cambrey AD, Occleston NL, Garrett Q, Tarnuzzer RW, Schultz GS et al. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci 2003; 44: 1104–1110.
Fini ME, Girard MT . Expression of collagenolytic/gelatinolytic metalloproteinases by normal cornea. Invest Ophthalmol Vis Sci 1990; 31: 1779–1788 (published erratum appears in Invest Ophthalmol Vis Sci 1990; 31: 2229).
Matsubara M, Girard MT, Kublin CL, Cintron C, Fini ME . Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodelling cornea. Dev Biol 1991; 147: 425–439.
Kenney MC, Chwa M, Alba A, Saghizadeh M, Huang ZS, Brown DJ . Localisation of TIMP-1, TIMP-2, TIMP-3, gelatinase A and gelatinase B in pathological human corneas. Curr Eye Res 1998; 1998: 238–246.
Ottino P, Taheri F, Bazen HE . Platelet-activating factor induces the gene expression of TIMP-1, -2, and PAI-1: imbalance between the gene expression of MMP-9 and TIMP-1 and -2. Exp Eye Res 2002; 74: 393–402.
Kjeldsen L, Sengelov H, Lollike K, Nielsen MH, Borregaard N . Isolation and characterization of gelatinase granules from human neutrophils. Blood 1994; 83: 1640–1649.
Girard MT, Matsubara M, Kublin C, Tessier MJ, Cintron C, Fini ME . Stromal fibroblasts synthesize collagenase and stromelysin during long-term tissue remodeling. J Cell Sci 1993; 104: 1001–1011.
Daniels JT, Limb GA, Saarialho-Kere U, Murphy G, Khaw PT . Human corneal epithelial cells require MMP-1 for HGF-mediated migration on collagen I. Invest Ophthalmol Vis Sci 2003; 44: 1048–1055.
Matsubara M, Zieske JD, Fini ME . Mechanism of basement membrane dissolution preceding corneal ulceration. Invest Ophthalmol Vis Sci 1991; 32: 3221–3237.
Fini ME, Parks WC, Rinehart WB, Girard MT, Matsubara M, Cook JR et al. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am J Pathol 1996; 149: 1287–1302.
Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA et al. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem 2002; 18(277): 2065–2072.
Jain S, Khoury JM, Chamon W, Azar DT . Corneal light scattering after laser in situ keratomileusis and photorefractive keratectomy. Am J Ophthalmol 1995; 120: 532–534.
Hersh PS, Brint SF, Maloney RK, Durrie DS, Gordon M, Michelson MA et al. Photorefractive keratectomy versus laser in situ keratomileusis for moderate to high myopia. A randomized prospective study. Ophthalmology 1998; 105: 1512–1522.
Park CK, Kim JH . Comparison of wound healing after photorefractive keratectomy and laser in situ keratomileusis in rabbits. J Cat Refract Surg 1999; 25: 842–850.
Tuft SJ, Zabel RW, Marshall J, Corneal repair following keratectomy . A comparison between conventional surgery and laser photoablation. Invest Ophthalmol Vis Sci 1989; 30: 1769–1777.
Nishida T, Tanaka T . Extracellular matrix and growth factors in corneal wound healing. Curr Opin Ophthalmol 1996; 7: 2–11.
Fini ME . Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retinal Eye Res 1999; 18: 529–551.
Tuft SJ, Gartry DS, Rawe IM, Meek KM . Photorefractive keratectomy: implications of corneal wound healing. Br J Ophthalmol 1993; 77: 243–247.
Seiler T, Holschbach A, Derse M, Jean B, Genth U . Complications of myopic photorefractive keratectomy with the excimer laser. Ophthalmology 1994; 101: 153–160.
Corbett MC, Prydal JI, Verma S, Oliver KM, Pande M, Marshall J . An in vivo investigation of the structures responsible for corneal haze after photorefractive keratectomy and their effect on visual function. Ophthalmology 1996; 103: 1366–1380.
Brown RA, McFarland CD . Regulation of growth plate cartilage degradation in vitro: effects of calcification and a low molecular weight angiogenic factor (ESAF). Bone Miner 1992; 17: 49–57.
Maddox PH, Jenkins D . 3-Aminopropyltriethoxysilane (APES): a new advance in section adhesion. J Clin Pathol 1987; 40: 1256–1257.
Hembry RM, Murphy G, Cawston TE, Dingle JT, Reynolds JJ . Characterisation of a specific antiserum for mammalian collagenase from several species: Immunolocalisation of collagenase in rabbit chondrocytes and uterus. J Cell Sci 1986; 81: 105–123.
Hipps DS, Hembry RM, Docherty AJ, Reynolds JJ, Murphy G . Purification and characterisation of human 72-kDa gelatinase (type IV collagenase) Use of immunolocalisation to demonstrate the non-coordinate regulation of the 72-kDa and 95-kDa gelatinases by human fibroblasts. Biol Chem Hoppe Seyler 1991; 372: 287–296.
Murphy G, Hembry RM, Reynolds JJ . Characterisation of a specific antiserum to rabbit stromelysin and demonstration of the synthesis of collagenase and stromelysin by stimulated rabbit articular chondrocytes. Coll Relat Res 1986; 6: 351–363.
Murphy G, Ward R, Hembry RM, Reynolds JJ, Kuhn K, Tryggvason K . Characterisation of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem J 1989; 258: 463–472.
Gallegos NC, Smales C, Savage FJ, Hembry RM, Boulos PB . The distribution of matrix metalloproteinases and tissue inhibitor of metalloproteinases in colorectal cancer. Surg Oncol 1995; 4: 111–119.
Vaalamo M, Weckroth M, Puolakkainen P, Kere J, Saarinen P, Lauharanta J et al. Patterns of matrix metalloproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol 1996; 135: 52–59.
Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC et al. The activity of Collagenase-1 is required for keratinocyte migration on a type I collagen Matrix. J Cell Biol 1997; 137: 1445–1457.
Madlener M, Parks WC, Werner S . Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res 1998; 242: 201–210.
Azar DT, Pluznik D, Jain S, Khoury JM . Gelatinase B and A expression after laser in situ keratomileusis and photorefractive keratectomy. Arch Ophthalmol 1998; 116: 1206–1208.
Yu Q, Stamenkovic I . Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumour invasion and angiogenesis. Genes Dev 2000; 14: 163–176.
Lu PC, Ye H, Maeda M, Azar DT . Immunolocalisation and gene expression of matrilysin during corneal wound healing. Invest Ophthalmol Vis Sci 1999; 40: 20–27.
Kure T, Chang JH, Kato T, Hernandez-Quintela E, Ye H, Lu PC et al. Corneal neovascularisation after excimer keratectomy wounds in matrilysin-deficient mice. Invest Ophthalmol Vis Sci 2003; 44: 137–144.
Ye HQ, Maeda M, Yu FS, Azar DT . Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Invest Ophthalmol Vis Sci 2000; 41: 2894–2899.
Acknowledgements
This work was supported in part by a BUPA scholarship, Moorfields Eye Hospital locally organized research and the Peel Research Trust. Professor G Murphy generously provided the MMP antibodies. Dr F Savage provided technical advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mulholland, B., Tuft, S. & Khaw, P. Matrix metalloproteinase distribution during early corneal wound healing. Eye 19, 584–588 (2005). https://doi.org/10.1038/sj.eye.6701557
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701557
Keywords
This article is cited by
-
Combined effects of interleukin-1β and cyclic stretching on metalloproteinase expression in corneal fibroblasts in vitro
BioMedical Engineering OnLine (2016)
-
Matrix metalloproteinases and epidermal wound repair
Cell and Tissue Research (2013)