Abstract
Aim
We used a retinal tomographic analyser to study the profile of the retinal surface in patients with stage 3 and 4 idiopathic macular holes, to attempt to elucidate the direction of forces present.
Methods
The Heidelberg retina tomograph was used to acquire a three-dimensional tomographic image of the macula in each eye of 21 consecutive patients with full thickness macular hole.
Results
The surface profile showed an elevated rim around the 24 macular holes imaged, with a gently sloping outside edge and a steeply sloping inside edge. In addition, a ring of elevated tissue around the edge of the hole was observed in all the holes and also in two of the fellow ‘normal’ eyes. This ring of elevated tissue was presumed to represent a ring of persistent vitreo-retinal traction around the fovea in the presence of a perifoveal posterior vitreous detachment. This is consistent with antero-posterior traction persisting in stage 3 and 4 full thickness macular holes. The mean ring diameter was 480 μm, when present in the fellow eye but was 950 μm in the presence of a macular hole, which we argue is suggestive of centrifugal displacement of retinal tissue on the formation of a stage 3 macular hole and provides evidence for tangential traction.
Conclusion
We suggest that antero-posterior traction forces are the primary cause of full thickness macular holes, with these forces persisting in stage 3 and 4 macular holes, while tangential forces serve to enlarge the hole at this later stage.
Similar content being viewed by others
Introduction
For more than a decade, it has been known that surgery is effective in restoring normal anatomy and near normal visual function,1, 2, 3, 4, 5, 6 in idiopathic full thickness macular holes. Surgical techniques continue to evolve and for many now include peeling of the internal limiting membrane, following removal of the posterior vitreous.7
The mechanism of macular hole formation, however, remains unclear. Paradoxically, as surgery has evolved to include the removal of epiretinal elements that would produce tangential traction on the fovea, the widely accepted theory of tangential traction,8, 9, 10 as the cause of idiopathic macular hole, has been brought into question.
The advent of new techniques in macular imaging and consequent improved imaging of the posterior vitreous face has shown that patients in the early stages of macular hole formation have a localised detachment of the posterior vitreous, with persistent attachment of the vitreous at the fovea.11, 12, 13, 14 This finding has been confirmed using a combination of bimicroscopy, ultrasonography, and careful examination of the posterior vitreous at the time of vitrectomy.15 The perifoveal posterior vitreous detachment with subsequent antero-posterior traction at the fovea is felt to be the primary event in macular hole development. It is proposed that this traction leads to first foveal splitting11, 12, 16, 17 and ultimately to full-thickness dehiscence.
There is less evidence regarding the tractional forces acting on macular holes in the later stages. Unoperated holes have been shown to progress slowly in size18 and it is proposed that tangential traction is predominant in enlarging and maintaining the hole, possibly by myofibroblastic contraction within a mild epiretinal membrane.7
We used a retinal tomographic analyser to study the profile of the retinal surface in patients with stage 3 and 4 idiopathic macular holes, to attempt to elucidate the direction of forces present.
Patients and methods
A total of 21 consecutive patients attending our vitreoretinal clinic with idiopathic macular hole underwent assessment, including Snellen visual acuity, fundus bimicroscopy and retinal tomography.
A diagnosis of macular hole was made by the appearance of a full thickness defect surrounded by a cuff of elevated retinal tissue. Assessment of the hole appearance and vitreous state allowed staging of the macular hole according to Gass's clinical classification.8, 10 A posterior vitreous detachment was assumed to be present where a Weiss ring was observed.
The Heidelberg retina tomograph (HRT) (Heidelberg Engineering, Heidelberg, Germany) was used to acquire a three-dimensional tomographic image of the macula in each eye. The HRT is a scanning laser ophthalmoscope (SLO) mounted on a stand and manipulated similarly to a fundus camera. It uses a diode laser of wavelength 670 nm and a retinal irradiance of 0.5 mW cm−1. A total of 32 consecutive two-dimensional (x- and y axis) optical sections, each comprising 256 × 256 pixels, were recorded at a repetition rate of 20 Hz. A scan field of 20° was selected to ensure that the entire ring of thickened retinal tissue surrounding the macular hole was encompassed. The 32 scans were equally spaced along the optical (z) axis. Scan depth was varied according to the depth of the macular hole. The 32 section images were than aligned to negate any eye movement that had occurred during image capture and to create a three-dimensional image.
HRT software (version 2.01) was used for topographical data analysis.
Results
We analysed data on 24 eyes with full thickness macular holes and 18 clinically normal fellow eyes.
In all 14 of the patients were female and seven were male, and their mean age was 69 years (range 55–84 years).
The clinical diagnosis was stage 3 in 18 eyes and stage 4 in five eyes by bimicroscopic assessment.
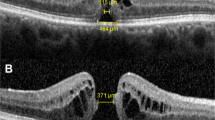
Qualitative analysis showed that the SLO appearance of the holes was similar to those of previous reports, having a central round depression with a concentric raised annular area with ill defined margins (Figures 1 and 2). We found the annular area around the hole to be sloped to give the appearance of a volcano with the macular hole as the crater. In addition, a ring of elevated tissue, outside but concentric to the edge of the hole, was observed on the tomographs in all the holes (Figures 1, 2 and 3) and in two of the fellow ‘normal’ eyes. This ring of elevated tissue was not detectable clinically and has not been reported by others.
Patient C. HRT colour image and surface profile through the centre of a left stage 3 macular hole. The red arrows mark the cross-section through the elevated ring we find concentric with, but slightly larger than the macula hole and lying at a slightly elevated position relative to the surrounding retinal surface.
Patient C. ‘Pseudo-3D’ reconstruction by the HRT software. (a) An oblique view of the macular hole demonstrates the volcano shaped profile of the hole and surrounding retina and the sharply elevated ring of tissue concentric with, but slightly larger than the hole. (b) A sagittal view of the macular hole shows the gently sloping rim of elevated retinal tissue surrounding the hole and the sharply elevated ring at the edge of the hole.
Further examples of the surface profile of full thickness macular holes and ‘pseudo-3D’ reconstruction with the HRT. The arrows indicate the elevated ring. (a) and (d) Patient L. Left stage 4 macular hole. (b) and (e) Patient P. Left stage 3 macular hole. (c) and (f) Patient S. Right stage 3 macular hole.
Results from quantitative analysis of the tomographs are given in Table 1.
The mean diameter of the macular holes was 550 μm (SD 120 μm), measured using the HRT software, horizontally from edge to edge at the midpoint of the hole. The mean diameter of the ring of elevated tissue in the presence of a macular hole was 950 μm (SD 180 μm), measured horizontally from peak to peak at the midpoint of the hole. The mean diameter of the ring of elevated tissue when present in the fellow eye was 480 μm (SD 10 μm). The mean elevation of the ring of tissue was 110 μm (SD 50 μm), measured from the retinal surface to the maximum height of the ring.
Discussion
New imaging techniques including optical tomography (OCT), the retinal thickness analyser, and the SLO are leading us to an improved understanding of the pathogenesis of idiopathic macular hole. This study examined the retinal profile of patients with full thickness macular holes with scanning laser tomography. It has been verified that scanning laser tomography can demonstrate layers otherwise difficult to examine, such as the posterior vitreous and inner retinal layers.19
In all cases clinically diagnosed as full thickness macular holes, the surface profile produced by the scanning laser tomograph showed an elevated rim around the macula hole, as previously documented by others.16, 20, 21, 22 We describe that the surface of the elevated, rim is not uniformly elevated but is found to be shaped like a volcano, with a gently sloping outside edge and a steeply sloping inside edge. This profile is also seen in the images of published studies using the SLO.20, 22
We also describe a ring, concentric with, but slightly larger than, the macula hole and lying at a slightly elevated position relative to the surrounding retinal surface. The ring was found in all patients with full thickness macular holes and in two fellow eyes.
The imaged ring must represent either sharply elevated retina or a ring of vitreous. Our images do not allow us to differentiate further but in either case would suggest that there is persistent attachment between the vitreous and the retina at this site, while there is detachment of the vitreous elsewhere in the perifoveal region. The ring is always found within 200 μm of the surrounding retinal surface and is therefore not likely to represent a detached vitreous, which has been shown to be 200–500 μm above the retinal surface in stage 3 macular holes.12
Others have imaged a similar feature using OCT. Spaide et al23 studied eyes with premacular hole states, in which they found a perifoveal posterior vitreous detachment. They showed persistent attachment of the vitreous in rings around the fovea and interestingly found that the degree of foveal distortion was linked to the diameter of the attachment.
Gaudric et al12 also used OCT to examine patients with a range of macular hole states. While they showed persistent perifoveal vitreous attachment in both impending macular holes and in stage 2 holes, they did not show any vitreoretinal adherence around the stage 3 macular hole. This led them to postulate that while antero-posterior forces are responsible for exerting traction on the foveolar centre and producing stage 1 and 2 macular holes, by stage 3, the hyaloid is completely detached from the macular.
Our findings, while adding to the evidence for antero-posterior traction in early macular hole formation, would also suggest that the vitreoretinal attachment persists in stage 3 holes. Furthermore, five eyes in our study were judged to have a complete posterior vitreous detachment by the presence of a Weiss ring on fundus bimicroscopy, yet still displayed the ring as a sign of vitreo-retinal attachment. This is consistent with the findings of Kishi et al.24 On scanning electron microscopy, they demonstrated remnants of posterior vitreous membrane still attached around the fovea in 26 of 59 autopsy eyes with spontaneous vitreous detachment. The remnants appeared as disc shaped membranes or rings of two differing sizes, 500 and 1500 μm.
It seems likely that the persistent vitreo-retinal attachment we image represents a particularly strong adhesion at the site of known maximum vitreo-retinal attachment at the macula,25 which persists despite posterior vitreous detachment. This anatomical variation would represent a significant risk factor for macular hole formation26 and account for its presence in all the macular holes in our study.
The evidence for antero-posterior traction in early macular hole states is compelling, and includes evidence of the early formation of foveal splitting or cyst formation and later full thickness tissue loss.11, 12, 16, 17 Immunohistochemistry shows a variable number of photoreceptors present in 67% of the resulting opercula27 and lends further evidence for the presence of antero-posterior traction in macular hole formation.
That antero-posterior traction continues during stage 3 and 4 macular holes is also consistent with some already described features. Kishi et al16 demonstrated radial retinal folds, with the SLO, in stage 3 holes. Striae in this direction are consistent with antero-posterior traction, and the fact that they resolved only after vitrectomy surgery suggests that the traction is persistent after hole formation.
While our study provides evidence for continuing antero-posterior traction in stage 3 and 4 holes, we also suggest that there is a component of tangential traction at this stage causing centrifugal displacement of retinal tissue on development of a stage 3 macular hole. The diameter of the ring we imaged in the two fellow eyes was 470 and 490 μm. This is consistent with the diameter of the ring of maximum vitreo-retinal attachment at the macula,25 which is approximately 500 μm and corresponds to the smaller of the two rings described by Kishi et al.24 However, in eyes with stage 3 and 4 macular holes the mean diameter of the ring was 950 μm (SD 180 μm). This is again comparable, if we assume tangential displacement of the ring on development of the macular hole. We have previously described two patients, who each had a stage 2 macular hole in one eye and a stage 3 hole in the other. In these patients, the ring of traction was visible in each eye and was 200–300 μm larger around the stage 3 hole,28 again suggestive that there is centrifugal displacement, given that the diameter of the ring of maximum vitreo-retinal attachment at the macula would be expected to be equal in fellow eyes. These findings are consistent with objective evidence of radial centrifugal photoreceptor displacement using perimetric techniques.29 This movement cannot be explained without a component of tangential traction.
We suggest that antero-posterior traction forces are the primary cause of full thickness macular holes, with these forces persisting in stage 3 and 4 macular holes, while tangential forces serve to enlarge the hole at this later stage.
The mechanism of macular hole formation is yet to be fully elucidated and further research is still required before we can fully understand the pathogenesis and further progress in the treatment of this enigmatic condition.
References
Wendel RT, Patel AC, Kelly NE, Salzano TC, Wells JW, Novack GO . Vitreous surgery for macular holes. Ophthalmology 1993; 100: 1671–1676.
Kim JW, Freeman WR, Azen SP, el-Haig W, Klein DJ, Bailey IL . Prospective randomised trial of vitrectomy or observation for stage 2 macular holes. Am J Ophthalmol 1996; 121: 605–614.
Gregor ZJ . Surgery for idiopathic full-thickness macular holes. Eye 1996; 10: 685–690.
Freeman WR, Azen SP, Kim JW, el-Haig W . Vitrectomy for the treatment of full- thickness stage 3 or 4 macular holes. Results of a multicentered randomised clinical trial. The vitrectomy for the treatment of macular hole study group. Arch Ophthalmol 1997; 115: 11–21.
Benson WE, Cruickshanks KC, Fong DS, Williams GA, Bloome MA, Frambach DA et al. Surgical management of macular holes: a report by the American Academy of Ophthalmology. Ophthalmology 2001; 108: 1328–1335.
Kang HK, Chang AA, Beaumont PE . The macular hole: report of an Australian surgical series and meta-analysis of the literature. Clin Exp Ophthalmol 2000; 28: 298–308.
Mester V, Kuhn F . Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol 2000; 129: 769–777.
Gass JDM . Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol 1988; 106: 629–639.
Johnson RN, Gass JDM . Observations, stages of formation, and implications for surgical intervention. Ophthalmology 1988; 95: 917–924.
Gass JDM . Reappraisal of bimicroscopic classification of stages of development of a macular hole. Am J Ophthalmol 1995; 119: 752–759.
Hee MR, Puliafito CA, Wong C, Duker JS, Reichel E, Schuman JS et al. Optical coherence tomography of macular holes. Ophthalmology 1995; 102: 748–756.
Gaudric A, Haouchine B, Massin P, Paques M, Blain P, Erginay A . Macular hole formation: new data provided by optical coherence tomography. Arch Ophthalmol 1999; 117: 744–751.
Chauhan DS, Antcliffe RJ, Rai PA, Williamson TH, Marshall J . Papillofoveal traction in macular hole formation: the role of optical coherence tomography. Arch Ophthalmol 2000; 118: 32–38.
Kishi S, Takahashi H . Three-dimensional observations of developing macular holes. Am J Ophthalmol 2000; 130: 65–75.
Johnson MW, Van Newkirk MR, Meyer KA . Perifoveal vitreous detachment is the primary pathogenic event in idiopathic macular hole formation. Arch Ophthalmol 2001; 119: 215–222.
Kishi S, Kamei Y, Shimizu K . Tractional elevation of Henle's fiber layer in idiopathic macular holes. Am J Ophthalmol 1995; 120: 486–496.
Asrani S, Zeimer R, Goldberg MF, Zou S . Serial optical sectioning of macular holes at different stages of development. Ophthalmology 1998; 105: 66–77.
Casuso LA, Scott IU, Flynn HW, Gass JD, Smiddy WE, Lewis ML et al. Long-term follow-up of unoperated macular holes. Ophthalmology 2001; 108: 1150–1155.
Varano M, Billi B, Scassa C, Rossi T, Stirpe M . Scanning laser ophthalmoscopy in the early diagnosis of vitreoretinal interface syndrome. Retina 1997; 17: 300–305.
Bartsch DU, Intaglietta M, Bille JF, Dreher AW, Gharib M, Freeman WR . Scanning laser tomographic analysis of the retina in eyes with macular hole formation and other focal macular diseases. Am J Ophthalmol 1989; 108: 277–287.
Beausencourt E, Elsner AE, Hartnett ME, Trempe CL . Quantitative analysis of macular holes with scanning laser tomography. Ophthalmology 1997; 104: 2018–2029.
Hudson C, Charles SJ, Flanagan JG, Brahma AK, Turner GS, McLeod D . Objective morphological assessment of macular hole surgery by scanning laser tomography. Br J Ophthalmol 1997; 81: 107–116.
Spaide RF, Wong D, Fisher Y, Goldbaum M . Correlation of vitreous attachment and foveal deformation in early macular hole states. Am J Ophthalmol 2002; 113: 226–229.
Kishi S, Demaria C, Shimizu K . Vitreous cortex remnants at the fovea after spontaneous vitreous detachment. Int Ophthalmol 1986; 9: 253–260.
Foos RY . Vitreoretinal juncture; topographical variations. Invest Ophthalmol 1972; 11: 801–808.
Johnson MW . Improvements in the understanding and treatment of macular hole. Curr Opin Ophthalmol 2002; 13: 152–160.
Ezra E, Farriss RN, Possin DE, Aylward WG, Gregor ZJ, Luthert PJ et al. Immunocytochemical characterisation of macular hole opercula. Arch Ophthalmol 2001; 119: 223–231.
Woon H, Bishop F . Evidence for centrifugal displacement of tissue from scanning laser tomographs of eyes with full thickness macula holes. Klin Monatsbl Augenheikd 1999; 215 (Suppl 2): 6.
Jensen OM, Larsen M . Objective assessment of photoreceptor displacement and metamorphopsia: a study of macular holes. Arch Ophthalmol 1998; 116: 1303–1306.
Author information
Authors and Affiliations
Corresponding author
Additional information
Proprietary Interests/research funding: none
This work was originally presented at the Congress of the Royal College of Ophthalmologists, 1999.
Rights and permissions
About this article
Cite this article
Bishop, F., Walters, G., Geall, M. et al. Scanning laser tomography of full thickness idiopathic macular holes. Eye 19, 123–128 (2005). https://doi.org/10.1038/sj.eye.6701432
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701432
Keywords
This article is cited by
-
Is axial length a risk factor for idiopathic macular hole formation?
International Ophthalmology (2012)