Abstract
Purpose To compare selective laser trabeculoplasty (SLT) with conventional argon laser trabeculoplasty (ALT) in terms of hypotensive efficacy, anterior chamber inflammation, and pain reported by the patients treated.
Methods A prospective study performed on 40 consecutive patients. Group I (n=20): SLT 180°. Group II (n=20): ALT 180°. Intraocular pressure, flare (Laser-Flare-Meter, Kowa FM-500, Japan), and pain (Visual Analogue Scale) were measured before treatment and 1 h, 24 h, 1 week, and 1, 3 and 6 months after treatment. Statistically significant differences were determined by an independent-sample Student's t-test.
Results At 6 months after treatment, pressure reduction was similar in both groups: SLT 22.2% (range 0–36.3%) and ALT 19.5% (range 0–30.2%), P=0.741. The energy released during treatment was significantly lower in SLT (48.3 SD 7.4 mJ) than in ALT (4321 SD 241.7 mJ), P<0.001. At 1 h after treatment, anterior chamber flare was also lower in SLT (13.3 SD 6.3 vs 20.7 SD 7.4 photons/ms), P=0.003. Pain reported by the patients during the treatment was significantly lower in SLT (2.0 SD 0.7 vs 4.3 SD 1.3), P<0.001.
Conclusions The hypotensive efficacy of both lasers at the end of follow-up was similar. The energy released during treatment and inflammation produced in the anterior chamber in the immediate postoperative period were significantly lower for SLT. The SLT procedure was better tolerated, producing less discomfort during treatment than conventional trabeculoplasty with argon.
Similar content being viewed by others
Introduction
Since its development, argon laser trabeculoplasty has been a useful tool for the treatment of open-angle glaucoma,1 both as a first therapeutic option even ahead of medical treatment and for glaucomas that cannot be controlled with topical treatment, as a previous step to the surgical intervention.2 Although its action mechanism is not well established, two hypotheses have been considered (mechanical and cellular) to explain the increased outflow of aqueous humour through the trabecular meshwork.3,4,5 The coagulation effect of the laser and the subsequent scarring that occurs in the trabeculum are thought to be the main responsible factors for the loss of treatment efficacy over time and for poor retreatment results.6,7
In 1998, Mark Latina published his first trabeculoplasty results8 using a pulsed Nd-YAG laser of 532 nm, which seems to act selectively on the pigmented cells of the trabeculum, respecting adjacent tissues without the coagulation harm that has been associated with the argon laser.9,10 This procedure is called selective laser trabeculoplasty. The action mechanism of this laser has not been completely explained. Since damage to the trabecular meshwork after treatment seems to be minimum compared to that experienced by argon laser-treated patients,11 the cell theory is more feasible than the mechanical theory. The energy released in these types of treatment is approximately 80 times less than for conventional treatment since it uses energies around 0.7 mJ and exposure times in the range of nanoseconds. According to Latina and Park,12 this exposure time would permit the energy to be selectively captured by the pigmented cells of the trabeculum, not affecting adjacent tissues and also avoiding heat diffusion from the pigmented cells to the rest of the trabecular structures, as that occurs in the case of the argon laser. The spot size used is pre-established at 400 μm, which is that required to maintain the low fluency (energy/area) needed for the selectivity of this laser.12
The following study was designed to determine if the lower amount of energy released in selective laser trabeculoplasty (SLT) could lead to less inflammation in the anterior chamber during the immediate postoperative period, and if this may in turn have an effect on the pain suffered by the patients and on postoperative pressure spikes compared to conventional treatment.
Materials and methods
This prospective study was performed on 40 eyes of 40 consecutive patients with early to moderate open-angle glaucoma that was poorly controlled by medical treatment (IOP>21 mmHg) and that had not been previously treated with laser or filtering surgery. Patients with pseudoexfoliative or pigmentary glaucoma were not included. Informed consent was obtained from each patient prior to treatment. The trabeculoplasty was performed in every case by the same ophthalmologist (JFG) and both the pre- and postoperative examinations were carried out by the same person who was blind to the type of laser used (JMC).
Patients in the SLT group (n=20) underwent SLT (Coherent Selecta 7000, Coherent Inc., Palo Alto, CA, USA) at the inferior 180°, with a spot size of 400 μm, exposure time of 3 ns and initial energy of 0.8 mJ in every case, which was increased by steps of 0.1 mJ until a visible effect was observed in the trabecular meshwork, at which time it was decreased by 0.1 mJ until treatment was completed. The argon laser trabeculoplasty (ALT) group patients (n=20) underwent an (ALT) (Visulas 532, Carl Zeiss Inc, Germany) on the inferior 180° applying sufficient power to obtain a visible effect in the trabecular meshwork (blanching or occasional bubble formation) with a 50 μm spot size and an exposure time of 0.1 s.
After treatment, all the patients were treated with topical fluorometholone (FML®, Allergan S.A., Madrid, Spain), administered three times a day for 1 week. Alpha agonists were not given in the immediate postoperative period. The hypotensive topical medication that the patients received before treatment was maintained during the 6 months of study to avoid changes that could lead to error in the analysis of intraocular pressure IOP after trabeculoplasty.
The patients were examined the day before the treatment and 1 h, 24 h, 1 week, and 1, 3 and 6 months after treatment. All tests were performed at 0900±1 h to reduce diurnal variation. In each examination, IOP was determined with an applanation tonometer and flare in the anterior chamber was measured using a laser flare meter (Kowa FM-500, Kowa Company Limited, Japan). The laser flare meter makes it possible to measure inflammation objectively in the anterior chamber.13 These measurements are based on the diffraction produced in the anterior chamber by a helium–neon laser beam of constant power. Flare intensity is proportional to the amount of protein in the anterior chamber14 and thus is an indirect indicator of the effect of the laser on the blood–aqueous barrier. Measured flare values are expressed in photon counts per millisecond given by the instrument. In the immediate postoperative check-up and at 24 h, a 0-to-10 visual analogue scale15,16,17 (VAS, UPSA Institute) was used for the patients to determine objectively the degree of pain suffered during treatment and the first postoperative hours. The patients were also asked questions of the type: how much pain or discomfort did treatment cause? or what type of discomfort did you feel at home during the first 24 h of treatment? Patients were asked to slide a marker along one side of a scale that shows a bar that gets progressively wider such that the observer, blind to the type of laser used, can quantify the pain according to the numerical scale on the underside. Statistical analysis was performed using SPSS 11.0 for Windows (SPSS Inc.). The normality of the data was checked using the Kolmogorov–Smirnov test. Statistically significant differences were determined by an independent-sample Student's t test. A P-value of less than 0.05 was taken to denote statistical significance.
Results
The demographic characteristics of both groups are shown in Table 1. No significant complications were noted in either group. The mean number of laser impacts during treatment were 52.3 SD 12.1 in the SLT group and 56.2 SD 7.2 in the ALT group, there being no statistically significant differences (P=0.632) between the groups. The mean energy used in SLT was 0.9 SD 0.1 mJ and the mean power used in the ALT group was 768.9 SD 39.6 mW. The total mean energy released (mean energy released=mean power used per impact * number of impacts * exposure time) in each treatment was significantly lower in SLT (48.3 SD 7.4 mJ) than in ALT (4321 SD 241.7 mJ), P<0.001.
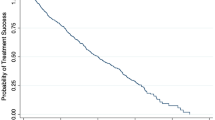
Mean IOP before treatment was 24.0 SD 4.7 mmHg in the SLT group and 23.6 SD 3.8 mmHg in ALT (P=0.75). At 1 h after treatment, mean IOP rise was lower in SLT (1.9 SD 3.4 mmHg) than in ALT (3.0 SD 4.8 mmHg), although this difference was not statistically significant (P=0.169). The hypotensive effect of each laser was equivalent during follow-up, no significant differences being found in any of the check-ups performed on the patients (Figure 1, Table 2). The mean percentage decrease in the SLT group at 6 months post-treatment was 22.2% (range 0–36.3%), and 19.5% in ALT (range 0–30.2), P=0.741. The percentage of responders (pressure decrease equal to or greater than 3 mmHg) was 80% (16 of 20 eyes) in SLT and 85% (17 of 20 eyes) in ALT. Among the responders, the mean percentage decrease in IOP was 26.7% (range 13.0% - 36.3%) in the SLT group and 21.8% (range 16.3–30.2%) in the ALT group (P=0.231).
At 1 h after treatment, anterior chamber flare was significantly lower in the SLT group (13.3 SD 6.3 photons/ms) than in the ALT group (20.7 SD 7.4 photons/ms), P=0.003. No differences were noted during the rest of follow-up (Figure 2, Table 3). Moderate correlation was found between the energy released during treatment and flare in the immediate postoperative period (r=0.56).
The pain reported by the patients during treatment (VAS) was also lower in the SLT group (2.0 SD 0.7 vs 4.2 SD 1.3), P<0.001, although these differences had disappeared in the 24-h assessment (Table 4).
Discussion
Only a few years ago, SLT emerged as one more tool for the treatment of patients with glaucoma.8 According to previous studies, the principal advantage of this laser over the conventional argon laser seems to be the smaller destructuration of the trabecular meshwork that occurs after treatment.11
Besides evaluating the hypotensive efficacy of SLT, the main objective of our study was to determine objectively whether the anterior chamber inflammation produced in response to SLT was significantly different from that produced after conventional treatment and whether this could lead to improved tolerability to treatment by the patient. To the best of our knowledge, there are no previous references on the objective measurement of anterior chamber inflammation in patients treated with SLT. Damji et al18 subjectively determined inflammation produced after both these treatments using the slit lamp. Damji reported that the degree of inflammation was significantly greater in patients subjected to SLT, and attributed this to the greater spot size. This would cause part of the impact to affect both the ciliary body region and the iris root, inducing greater inflammation during the first few hours of the postoperative period. In our patient series, we found that anterior chamber inflammation in the SLT group was significantly lower than in the argon laser-treated group during the initial postoperative hours. This could be due to the lower energy released during treatment with the Nd–YAG laser. It is likely that the subjective estimation of the number of anterior chamber cells does not adequately reflect alteration of the blood–aqueous barrier. It could be that instead, this method quantifies the detritus and pigment released in response to treatment and is not related to the inflammatory response. During the rest of the follow-up period, the differences between the two groups were not significant though the degree of inflammation in the SLT group never exceeded that of the argon laser-treated patients, contrasting with observations by Damji et al.18 Mermoud et al,19 after treating 71 eyes with ALT, found a greater inflammatory peak 48 h after treatment, which was accompanied by a greater pressure decrease at this stage of follow-up. His explanation for these events was based on the capacity of the trabecular meshwork to synthesize prostaglandins, which could act as mediators of the inflammation and, at the same time, would have a beneficial effect on IOP. The fact that our patients were treated from the onset with a glucocorticoid capable of inhibiting prostaglandin synthesis could explain why these findings were not observed in either of our two treatment groups.
The lower energy used in SLT could also mean less pain for patients during the procedure compared to argon laser treatment. Our patients showed significantly better tolerance to SLT only at the time of the treatment, since the pain suffered during the first 24 h was comparable in both the groups.
With regard to the hypotensive effect of both treatments, we found that both lasers in the short run can be considered comparable. We found no significant differences in this effect in any of the postoperative assessments. Damji also detected no differences between these lasers when he treated 36 eyes of 34 patients with a 6-month follow-up.18 If we compare the pressure decrease noted at 6 months to those reported by other authors, the mean decrease of 22.2% noted in our patients is similar to the 19.3% described by Damji et al,18 the 25% by Gracner20 and the 20.1% by Latina et al,8 yet higher than the 15.6% reported by Kim and Moon et al.21 Lanzetta et al22 obtained a mean pressure decrease of 39.9% in eight eyes of six patients with a 6 week follow-up after treating 360° of the iridocorneal angle in a single session. Our percentage of responders (80% with pressure decrease ≥3 mmHg) is similar to the 70% published by Latina et al8 using the same criterion.
The most serious complication of ALT is the occurrence of an IOP spike in the immediate postoperative period.23,24 According to the Glaucoma Laser Trial Research Group,25 over 30% of the patients treated showed a 5 mmHg or greater increase in IOP. After treating 84 eyes with ALT, Rosenblatt and Luntz26 noted a positive correlation between the IOP rise in the immediate postoperative period and the energy released during treatment. Our results show a lower IOP rise 1 h after treatment in the SLT group (1.9 SD 3.4 mmHg vs 3.0 SD 4.8 mmHg), corresponding to the lower amount of energy released (group I: 48.3 SD 7.4 mJ vs group II: 4321 SD 241.7 mJ). Only two patients in each group showed a significant increase in the IOP greater or equal to 5 mmHg during treatment. In all four patients, IOP had returned to normal at 24 h post-treatment without the need for additional treatment.
Based on our results, we can conclude that SLT is an effective and safe tool for the treatment of glaucomas that are poorly controlled by medical treatment. Selective trabeculoplasty is a simple technique to perform even by an inexperienced ophthalmologist, since the large size of the spot avoids the need to locate the impact in a specific zone of the trabecular meshwork. The lower energy released during treatment and less inflammation produced in response to treatment means it is better tolerated by the patient. Owing to the novelty of this technique, its middle or long-term hypotensive effects have not been totally well established. The repercussions that the lower alterations produced in the trabecular meshwork can have in the retreatments and if these can have better results than those obtained with the conventional treatment must be analysed with the passage of time.
References
Wise JB, Witter SL . Argon laser therapy for open angle glaucoma. Arch Ophthalmol 1979; 97: 319–322.
Glaucoma Laser Trial Research Group. The glaucoma laser trial (GLT) and glaucoma laser trial follow up study. Am J Ophthalmol 1995; 120: 718–731.
Wise JB . Glaucoma treatment by trabecular tightening with the argon laser. Int Ophthalmol Clin 1981; 21: 69–78.
Melamed S, Pei J, Epstein DL . Short term effect of argon laser trabeculoplasty in monkeys. Arch Ophthalmol 1985; 103: 1546–1552.
Gylbert CM, Brown RH, Lynch MG . The effect of argon laser trabeculoplasty on the rate of filtering surgery. Ophthalmology 1986; 93: 362–365.
Rodrigues MM, Spaeth GL, Donohoo P . Electron microscopy of argon laser therapy in phakic open angle glaucoma. Ophthalmology 1982; 89: 198–210.
Ticho U, Zauberman H . Argon laser application to the angle structures in the glaucomas. Arch Ophthalmol 1976; 94: 61–64.
Latina MA, Sibayan SA, Shin DH, Noecker RJ, Marcellino G . Q-switched 532 nm Nd–YAG laser trabeculoplasty (selective trabeculoplasty). A multicenter, pilot, clinical study. Ophthalmology 1998; 105: 2082–2090.
Alexander RA, Grierson I, Church WH . The effect of argon laser trabeculoplasty upon the normal human trabecular meshwork. Graefes Arch Clin Exp Ophthalmol. 1989; 227: 72–77.
Alexander RA, Grierson I . Morphological effects of argon laser trabeculoplasty upon the glaucomatous human meshwork. Eye 1989; 3: 719–726.
Kramer TR, Noecker RJ . Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasty in human eye bank eyes. Ophthalmology 2001; 108: 773–779.
Latina MA, Park C . Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW laser interactions. Exp Eye Res 1995; 60: 359–371.
Herbort CP, Bigar F, Pittet N . Usefulness of the Laser Flare Cell Meter (LFCM, Kowa FC-1000) for evaluating inflammation of the anterior chamber in clinical practice. Klin Monatsbl Augenheilkd 1991; 198: 470–473.
Ogawa T, Ohara K, Shimizu H . Correlation between total aqueous protein concentrations and photon counts in rabbits. Nippon Ganka Gakkai Zasshi 1990; 94: 1001–1006.
Bijur PE, Silver W, Gallagher EJ . Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med 2001; 8: 1153–1157.
Jensen MP, Turner JA, Romano JM, Fisher LD . Comparative reliability and validity of chronic pain intensity measures. Pain 1999; 83: 157–162.
Bardocci A, Lofoco G, Perdicaro S, Ciucci F, Manna L . Lidocaine 2% gel versus lidocaine 4% unpreserved drops for topical anesthesia in cataract surgery: a randomized controlled trial. Ophthalmology 2003; 110: 144–149.
Damji KF, Shah KC, Rock WJ, Bains HS, Hodge WG . Selective laser trabeculoplasty vs argon laser trabeculoplasty: a prospective randomised clinical trial. Br J Ophthalmol 1999; 83: 718–722.
Mermoud A, Pittet N, Herbort CP . Inflammation patterns after laser trabeculoplasty measured with the laser flare meter. Arch Ophthalmol. 1992; 110: 368–370.
Gracner T . Intraocular pressure response to selective laser trabeculoplasty in the treatment of primary open angle glaucoma. Ophthalmologica 2001; 215: 267–270.
Kim YJ, Moon CS . One year follow up of laser trabeculoplasty using Q-switched frequency doubled Nd-YAG laser of 523 nm wavelength. Ophthalmic Surg Lasers 2000; 31: 394–399.
Lanzetta P, Menchini U, Virgili G . Immediate intraocular pressure response to selective laser trabeculoplasty. Br J Ophthalmol 1999; 83: 29–32.
Ofner S, Van Buskirk EM, Samples JR . Medical therapy for the acute postoperative intraocular pressure rise following argon laser trabeculoplasty. Arch Ophthalmol. 1987; 105: 1476–1477.
Moulin F, Haut J, Le Mer Y, Vidal-Cherbonneau A . Adverse effects and complications of argon laser trabecular retraction: practical results. J Fr Ophtalmol 1987; 10: 773–776.
The Glaucoma Laser Trial. I. Acute effects of argon laser trabeculoplasty on intraocular pressure Glaucoma Laser Trial Research Group. Arch Ophthalmol. 1989; 107: 1135–1142.
Rosenblatt MA, Luntz MH . Intraocular pressure rise after argon laser trabeculoplasty. Br J Ophthalmol 1987; 71: 772–775.
Acknowledgements
This study was supported in part by the instituto de Salud Carlos III. Ayuda para el desarrollo de redes tematicas (C03/13). None of the authors has a financial interest in any product mentioned.
Author information
Authors and Affiliations
Corresponding author
Additional information
We transfer all copyright ownership to the Royal College of Ophthalmologists in the event this paper is published.
Rights and permissions
About this article
Cite this article
Martinez-de-la-Casa, J., Garcia-Feijoo, J., Castillo, A. et al. Selective vs argon laser trabeculoplasty: hypotensive efficacy, anterior chamber inflammation, and postoperative pain. Eye 18, 498–502 (2004). https://doi.org/10.1038/sj.eye.6700695
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6700695
Keywords
This article is cited by
-
Selective laser trabeculoplasty is safe and effective in patients previously treated with prostaglandin analogs: An evidence-based review
International Ophthalmology (2022)
-
Could adverse effects and complications of selective laser trabeculoplasty be decreased by low-power laser therapy?
International Ophthalmology (2019)
-
Efficacy of selective laser trabeculoplasty in primary angle closure disease
Eye (2018)
-
Corneal topographic alterations after selective laser trabeculoplasty
International Ophthalmology (2017)
-
Where does selective laser trabeculoplasty stand now? A review
Eye and Vision (2016)