Abstract
Background Disposable devices are increasingly becoming the preferred choice where possible in contact medical equipment.
Aim To evaluate the accuracy of the disposable applanation tonometer head as a potential substitute to the standard Goldmann applanation head.
Methods The study was prospective. The intraocular pressure recordings in 80 eyes of 42 patients were compared using the disposable and standard Goldmann applanator heads. The Bland and Altman method of assessing agreement between two methods of clinical measurement was used in the analysis.
Results The difference in the readings between the two types of tonometer heads was highly variable (mean difference=0.78 mm Hg, range=−1 to 11 mm Hg). This was because of the distortions on the applanating surface of the disposable device. When the readings associated with the defective heads were excluded, very strong agreement was obtained (mean=0.07 mm Hg, range=−1 to 2 mm Hg).
Conclusion Good agreement with standard Goldmann applanation is achieved with the disposable heads except where surface distortions induce significant errors. Careful inspection to ensure well-structured disposable units is imperative in disposable applanation tonometry.
Similar content being viewed by others
Introduction
Disposable devices are an alternative that are increasingly becoming the preferred option with regard to contact ophthalmic equipment, arising from the fear of transmission of various infections. Several reports are put forward on viral (herpes simplex virus, adenovirus, HIV, hepatitis), parasite (acanthamoeba), and prion (Creutzfeldt–Jakob Disease) related infections.1,2 Reusable devices have various recommendations towards their sterilization (depending on the material of the device), to eliminate the occurrence of these infections. However good these methods may be, the risk of infection can never be eliminated.3 Disposable devices may provide the solution to the infection problem, but have their own problems of standardization (especially for diagnostic measurement devices) and cost. The Medical Devices Agency recommends that ‘components of ophthalmic devices that touch the surface of the eye should be restricted to single patient use wherever practicable and where this does not compromise clinical outcome.’4
Applanation tonometry is the gold standard across the UK for the measurement of intraocular pressure (IOP) with Goldmann applanators being the most widely used. Since the process involves contact with the ocular surface, there is concern about the spread of communicable diseases. Until now, the applanator heads have been resterilized between patients by chlorhexidine wipes, isopropyl alcohol soaks, etc. This does not guarantee 100% safety3 and there is the risk of retaining some of the chemical disinfectant on the applanator surface which may damage the cornea. Disposable prism heads have proved a cost-effective option against these problems (60 pence/head). Studies performed have shown these heads to provide accuracy consistent with standard applanators (up to ±0.5 mmHg).
This report aims to assess the accuracy and reliability of the disposable device in comparison to the standard Goldmann applanator.
Materials and methods
This was a prospective study. A total of 80 eyes of 42 consecutively suitable patients were checked over a duration of 2 weeks. Patients excluded were children and those with corneal pathologies. Only one person was involved in the measurement process to eliminate user-related errors in variation between the two heads. Both types of heads (Standard Goldmann & Disposable) were used in every patient, alternating in consecutive patients to eliminate the error that could be introduced by the first measurement. IOP was checked on the slit lamp after instilling a drop of proxymetacaine hydrochloride 0.5% and fluorescein sodium 0.25% from a preservative-free single-dose minim. Repeated tonomtery was avoided5 and care was taken to ensure that the patient always looked straight ahead, and there was no external pressure on the eyeball. The slit-lamp breath shield was masked 6 so that the user could not assess the reading on the scale at any point. Another observer noted the readings during the measurement. The above precautions are in accordance with other similar comparison studies.7 All the disposable heads used were preserved.
The dispoasable applanation device used consists of a precision moulded holder and a disposable optical doubling applanating prism (see Figure 1). The manufacturer's leaflet declares the optical doubling effect to be within international standards. The holder with the prism mounted (see Figure 2) has the same mass as the standard Goldmann prism (1.65±0.5 g). The applanating surface of the disposable prism has the same area–diameter specifications as the standard Goldmann applanation head. Instructions for use are to remove the holder from the box and peel back the lid covering one of the disposable prisms in the box of 20. The holder is then pushed onto the prism still in its compartment, from behind, thereby avoiding contact with the applanating surface. There is an indexing key moulded into the prism which should align with the slot in the holder (Figure 1). The holder must be pushed completely on the prism. The holder prism complex is then fixed to the applanator just like a standard head and used. After use, the prism alone is disposed and the holder is reused in the same manner.
Results
Table 1 shows that IOPs dealt with were in the range of 9–23 mm Hg. The mean IOP was 15.96 mm Hg SD=2.9 (Goldmann) and 15.19 mm Hg SD=3.17 (disposable). It is apparent that the Goldmann was recording an average of 0.78 mm Hg SD=2.22 higher than the disposable. This is higher than that derived from other similar studies.7 What becomes evident is a high range of Goldmann vs disposable reading variation of up to 11 mmHg.
Careful examination of the disposable heads revealed that in five heads out of the 42 used, corresponding to 10 readings from the 80, there were distortions (dimpling) on the applanating surface (Figure 3). These were probably moulding distortions, and were causing false low recordings by the disposables.
This photograph has been taken with the disposable heads placed at the same angulation to the camera, so that the light reflected off the flat surfaces from both heads should be the same. The depression does not reflect light uniformly like the flat surface and hence the dark area is visible on the head on the left.
The parameters were reassessed by excluding the patients on whom the defective disposable heads were used (Table 2). The recorded IOP range was now 9–21 mm Hg. The mean IOP was 15.87 mmHg SD=2.82 (Goldmann) and 15.80 mmHg SD=2.89 (disposable). Now the Goldmann was recording an average of only 0.07 mmHg SD=0.60 higher than the disposable.
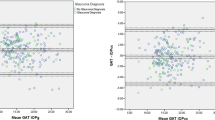
The data were further analysed using the method described by Bland and Altman8 for assessing agreement between the two methods of clinical measurement. Figure 4 shows the difference between the Goldmann and the disposable readings plotted against the average of the two readings. The thick black horizontal line represents the mean (0.78 mmHg), and the range of two SD from the mean is shown by the two thick grey lines (that is between −3.46 and 5.02). The scattering pattern is erratic, with the tendency being for the disposable to under-record. The defective disposable heads are responsible for this pattern of distribution.
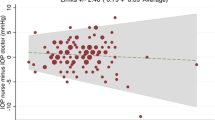
The analysis was repeated by removing the readings where the defective disposable heads were used (Figure 5). The value of the mean line (thick black) is 0.07 mmHg. The thick grey lines representing two SDs on either side are now −1.13 and 1.27. The distribution pattern is symmetrical and closer about the mean. Much better agreement between the two types of heads is revealed.
Discussion
This study shows that structural deformities in the disposable applanation devices have serious implications on the accuracy of intraocular pressure measurements. It proves imperative that every head is inspected in close detail for malformations before proceeding with disposable head use. The study also shows that when deformities are excluded, on average the disposable heads read only 0.07 mm Hg lower than the Goldmann heads, thus exhibiting very good agreement. The possible source of error in this determination of agreement would come from the fact that the 80 readings were in fact from 42 patients, giving rise to interclass correlation. Moreover, the results from multiple users have not been assessed and compared, and it is important to realize that results may differ for other observers. In addition, the defective heads were all detected by naked eye and slit-lamp examination of the surface. The effects of more microscopic variations need to be considered.
In keeping with the recommendations of the MDA,4 ophthalmologists should review their clinical practice and take measures to avoid risks of cross-infection. In tonometry, noncontact options have been considered in this regard, but devices such as the Pulsair tonometers showed deterioration in accuracy through repeated use, and regular recalibration is needed.9 Applanation, being the most reliable and followed method, has involved several disinfection methods to allow sterile usage. Chemical disinfection is the common method but incomplete removal of microorganisms,3 damage to the head, irritation to the ocular tissues, etc have proved disadvantages.10 The disposable silicone applanation shield has been offered as an option.10 However, studies have shown the shield to cause average over-readings of 1.9 mmHg.10 Moreover, the disposable shields cost over 70 pence per shield. The disposable prism since it has been introduced has been suggested to be the ideal solution.11 The style weight and design of the device correlates extremely well with the accepted Goldmann applanation devices and hence there is good agreement in the readings as well. The clear acrylic structure makes viewing of the fluorescein rings very bright and clear. Like any other disposable device, quality control in reproducibility of the device structure ensuring accuracy in measurements is vital. The large errors and potential misdiagnosis of IOPs are evident by the obvious flaws detected in the disposable devices in this report. It is highlighted that if at every use the disposable applanation device is thoroughly checked, good accuracy in IOP readings can be obtained and serious misreadings can be avoided.
References
Warren D, Nelson KE, Farrar JA, Hurwitz E, Heirholzer J, Ford E et al. A large outbreak of keratoconjunctivitis: problems in controlling nosocomial spead. J Infect Dis 1989; 160: 938–943.
Koo D, Bouvier B, Wesley M, Courtright P, Reingold A . Epidemic Keratoconjunctivitis in a university medical center ophthalmology clinic: need for re-evaluation of the design and disinfection of instruments. Infect Control Hosp Epidemiol 1989; 10: 547–552.
Smith CA, Pepose JS . Disinfection of tonometers and contact lenses in the office setting: are current techniques adequate? Am J Ophthalmol 1999; 127: 77–84.
Medical Devices Agency. MDA AN 1994(04), October 1999.
Gilchrist JM . On the precision and reliability of IOP measurements. Br J Ophthalmol 1996; 80: 586–587.
Gillow JT, Aggarwal R . Reducing bias during intra ocular pressure measurement. Br J Ophthalmol 1995; 79: 1057–1058.
Desai SP, Sivakumar P, Fryers PT . Evaluation of a disposable prism for applanation tonometry. Eye 2001; 15: 279–282.
Bland JM, Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; I: 307–310.
Atkinson PL, Wishart PJ, James JN, Vernon SA, Reid F . Deterioration in the accuracy of pulsair non contact tonometer with use: need for regular calibration. Eye 1992; 6: 530–534.
Maldonado MJ, Rodriguez Galietero A, Cano-Parra J, Menezo JL, Diaz-Llopis M . Goldmann applanation tonometry using sterile disposable silicone tonometer shields. Ophthalmology 1996; 103: 815–821.
Lueck CJ, Mc Ilwaine GG, Zeidler M . Creutzfeldt-Jakob disease and the eye. I. Background and patient management. Eye 2000; 14: 263–290.
Author information
Authors and Affiliations
Corresponding author
Additional information
Institutions to which this work was attributed Diana Princess of Wales Hospital Grimsby & Kent County Ophthalmic Hospital Maidstone
Rights and permissions
About this article
Cite this article
Goel, S., Chua, C., Dong, B. et al. Comparison between standard Goldmann applanation prism and disposable applanation prism in tonometry. Eye 18, 175–178 (2004). https://doi.org/10.1038/sj.eye.6700553
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6700553
Keywords
This article is cited by
-
Potential effects of systematic errors in intraocular pressure measurements on screening for ocular hypertension
Eye (2013)
-
Reliability of tonosafe disposable tonometer prisms: clinical implications from the Veterans Affairs Boston Healthcare System Quality Assurance Study
Eye (2011)
-
Comparison of Luneau SA disposable and Goldmann applanation tonometer readings
Eye (2007)
-
A comparison of clinical performance between disposable and Goldmann tonometers
Eye (2006)
-
Use of disposable prism tonometry in routine clinical practice
Eye (2005)