Abstract
Purpose To describe clinical and pathological features of Hydroview® intraocular lenses undergoing delayed surface opacification resulting in visual deterioration.
Methods Twenty one eyes which underwent uncomplicated phacoemulsification and Hydroview lens implantation with good visual recovery, presenting at 46–146 weeks post-surgery with visual deterioration and glare symptoms resulting from opacification of the implants, were included in the study. Twelve eyes had severe opacification, of which nine underwent intraocular lens exchange and three more are still awaiting surgery. The method of explantation is described. The explanted intraocular lenses were examined using light microscopy, scanning electron microscopy and x-ray microanalysis using a light element detector.
Results Light microscopy and scanning electron microscopy revealed diffuse granular deposits of approximately 5 μm diameter covering the optic surfaces but sparing the lens haptics. Light microscopic staining techniques and x-ray microanalysis confirm the major component of the deposits to be calcium phosphate salts.
Conclusions Late opacification of Hydroview intraocular lens implants is uncommon and aetiology seems to be multifactorial. Implant exchange is necessary to restore sight in some cases. As new materials are increasingly used it is important to highlight such unusual occurrences.
Similar content being viewed by others
Introduction
The use of foldable intraocular lens (IOL) implants during phacoemulsification cataract surgery has become increasingly popular. The three currently available materials for foldable IOL implants are silicone, acrylic and hydrogel. Dislocation or decentration and incorrect lens calculation are the principal complications leading to explantation of these lenses.1 Glare and optical aberrations encountered in some patients with multifocal or 3-piece acrylic lenses may also lead to explantation.
Clouding of IOL implants has resulted in few explantations but seems to have been a less common occurrence. Early fogging of silicone implants2 and late fogging of acrylic implants3 have been previously reported in sporadic cases. More recently,4,5,6 there have been a few reports of late surface opacification of hydrogel implants of different manufacturers from centres in Europe, Canada, Australia and the Far East. Most of the reported opaque hydrogel implants requiring explantation were in Hydroview® lens implant manufactured by Bausch and Lomb Surgical (Rochester, NY, USA).
We have been using Hydroview IOL implants in our department routinely since 1996. We encountered the first case of late opacification of the implant in late 1999. Since that time, we have seen a total of 21 cases. The opacification was severe and resulted in significant deterioration of vision in 12 eyes out of which nine underwent explantation. We describe below the patients, the technique of explantation and results of the analysis of the explanted lenses.
Methods
A total of 21 eyes underwent uncomplicated phacoemulsification surgery with Hydroview IOL (model H60M) implantation in the capsular bag through a small incision. All implants were packaged in the newer SureFold system introduced by Bausch and Lomb in 1997. Surgeries were performed by experienced surgeons in one centre under local anaesthesia between March 1998 and September 1999. All eyes were noted to have developed diffuse fine granular deposits on the surface of the IOL implant optic, at a mean interval of 101 weeks (range, 46–146) postoperatively. Pre- and postoperative visual acuities, other details of surgeries, and information related to the observation of opacification are summarised in Table 1.
In 12 cases (1–7, 13, 15–16, 18 and 21), the deterioration of vision and/or glare was significant, requiring exchange of the implant. The implants in three cases (6, 16, 21) have not yet been exchanged. All explanted lenses were divided in two, one half was placed in 4% Formaldehyde and the other half was transported in 2% Glutaraldehyde before further preparation for electron microscopy.
The remaining nine cases show mild surface opacification which is not visually significant. These patients remain under review, but none has been advised to have IOL implant exchange.
In cases 6 and 19, cataract surgery in the fellow eyes was performed 9 and 4 months later respectively, using Hydroview IOL implants which remain clear to date. In cases 10, 14 and 20, although cataract surgery with a Hydroview IOL in the fellow eyes was performed 12–16 months earlier, there are no signs of opacification.
Explantation
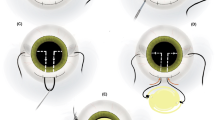
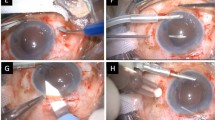
This was performed under local anaesthesia in all cases. The implant was removed through a scleral incision measuring 6 mm and replaced with a Poly(methylmethacrylate) implant in eight cases. The lens capsule was noted in all cases to be adherent to the anterior lens implant optic surface with a fine layer of epithelial cells extending from the edge of the capsulorhexis centrally. A 25-gauge needle was used to incise this membrane at the edge of the capsulorhexis and then with the aid of a viscoelastic, the implant was dialled out of the capsular bag.
Anterior vitrectomy was required in cases 5 and 6 where a YAG laser posterior capsulotomy was previously performed, and in case 2 where a posterior capsular tear was noted after removal of the implant. In one patient (case 3), the lens was divided in the anterior chamber with a lens cutter and removed through a small scleral incision, then a foldable silicone implant was injected into the capsular bag.
Pathology
The explanted lenses were fixed in formalin for at least 3 days and then embedded in methylmethacrylate resin. Using a glass knife, sections were cut for Haematoxylin and Eosin, Alizarin Red and von Kossa staining, using standard techniques.7
Scanning electron microscopy and X-ray microanalysis
All lenses were either routinely freeze-dried or critical-point dried through CO2, coated with carbon and examined in a Jeol 6100 scanning electron microscope (Japanese Electron Optics, Tokyo, Japan) operated at 10–20 KV. Images were collected on a Jeol Semafore digital imaging system. For element identification the rough surfaces of the lens were analysed in both spot and selected area mode using an Oxford instruments ISIS X-ray microanalysis system at 50 seconds livetime.
Results
Twenty-one patients (six male, 15 female) with a mean age of 80 years old (range, 67–91) had uncomplicated phacoemulsification cataract extraction and intraocular lens implantation with good initial visual recovery. Twelve eyes developed significant opacity of the IOL (Figure 1), resulting in reduced visual acuity or glare and were advised to undergo IOL exchange. The remaining patients did not develop significant symptoms. Cases 4, 6, 9 and 16 also had trabeculectomy for uncontrolled open angle glaucoma combined through the same site of small incision cataract surgery. Three patients were diabetics.
All implants used were Hydroview delivered in the new SureFold system introduced by the manufacturer in 1997 in which the lens holder also serves as a lens folder. The irrigating solution used intra-operatively was either BSS plus or BSS (Alcon Laboratories, Fort Worth, TX, USA). Viscoelastic used during surgery was Viscoat® (Alcon Laboratories) except in cases 7 and 13, where the viscoelastic used was not documented. None of the patients presenting with late opacification of the implant was known to suffer from any calcium metabolic disorders. One patient (case 16) was taking disodium etidronate for osteoporosis.
In five patients, the fellow eyes had Hydroview IOL implants, all of which were clear of any opacity at the last review. In three of these patients, the implants were delivered in the old packaging system which has not been involved with any reported surface opacification. However, in two patients, the implants used were delivered in the new SureFold system and yet both remain clear.
Pathology
Examination of explanted lenses revealed granular material covering both surfaces of the lens implant optic but not the implant haptic. All but one of the lenses showed a deposit of a dark blue/purple granular band over the lens surface with H&E stain, but did not show any deposition below the surface in the body of the lens itself. One lens showed patchy rather than confluent deposition. The Alizarin Red and von Kossa (Figure 2) stains both gave strong staining of the granular band, indicating it to contain calcium. There was no evidence of cellular infiltration accompanying the calcium deposition.
Scanning electron microscopy and X-ray microanalysis
This revealed diffuse granular deposits of approximately 5 μm diameter covering the optic surfaces but sparing the lens haptics and no cellular reaction (Figure 3). X-ray microanalysis revealed a significant calcium peak at 3.69 Kev and phosphorus peak at 2.01 Kev from the lens surface deposits (Figure 4).
Discussion
The optic of Hydroview IOL model H60M is a copolymer of 2-hydroxyethyl methacrylate and 6-hydroxyhexyl methacrylate with a small quantity of 1,6-hexanediol added as a crosslinker to impart dimensional stability and an added ultra-violet blocker. The haptics are poly(methyl-methacrylate) (PMMA) crosslinked with ethylene glycol dimethacrylate and appear blue from the incorporation of a dye. The two materials are joined together by allowing the haptic PMMA monomer to diffuse into the zero-water-containing (xerogel) lens body prior to polymerization of the PMMA. A one piece IOL is then lathe-cut from the xerogel/PMMA composite disk and the IOL shape is milled out of the blank, tumble polished, sterilized by steam autoclaving and delivered in the fully hydrated state in sterile water.
Ideally, the Hydroview IOL should be folded as soon as possible after removal from the package. This is achieved easily with a special forceps (hydrofolder), then the lens is grasped in the folded state with an inserting forceps. Implantation is possible through a 3.5–4 mm incision whilst in the folded state. In late 1997, the packaging of the IOL was modified and the new SureFold system was introduced. In this new package, the lens is laid on a folding platform, where after the lens is removed from the container, folding could be achieved by pressing on the lens holder without the need for the older style hydrofolder.
In late 1999 Bausch and Lomb became aware of reports of late clouding or fogginess of the lens post implantation associated with the new SureFold package. In some cases this led to clinically significant reduction in visual acuity resulting in replacement of the lens. Yag laser was ineffective in clearing the deposits from the surface of the lenses in the reported patients. Histopathological analysis of the lenses demonstrated irregular granular deposits on the surface of the optics which stained positive for calcium.4 Significant deposition of crystalline material on the surface of IOLs is uncommon but has been reported intra-operatively on silicone8 lenses and early in the postoperative period on hydrogel9 lenses. Similar calcium deposition associated with spoilation of soft contact lenses10 following extended wear has also been known for some time.
In our institution, Hydroview has been in use since 1996 and more than 5000 implants have been used to date. It is our experience that since the introduction of the new packaging (SureFold), 21 eyes developed late opacification of the implant between 46 and 146 weeks postoperatively, this was visually significant in 12 eyes requiring explantation. As of March 2001, Bausch and Lomb is aware of 309 cases of presumed opacification in 31 out of 3500 sites using the IOL worldwide, with 96 being clinically significant resulting in replacement.
Similar to a previous report,4 histopathological examination of the explanted lenses in our series demonstrated that the opacification is due to deposition of calcium on the surface of the optic, sparing the lens haptic and without involvement or structural change below the surface in the body of the lens itself. The ultra-structure and location of the opacity is clearly demonstrated in our series with scanning, and transmission electron microscopy and X-ray microanalysis confirmed the deposits to be mainly calcium and phosphorus.
We did not encounter any similar changes in eyes receiving Hydroview delivered in the older style package. Three eyes with opacified lenses had clear Hydroview lenses in the fellow eyes, though the clear lenses were delivered in the older style package. This, together with the absence of any reports of opacification associated with the older style packaging, points to a SureFold product factor resulting in (but not alone) initiating calcium deposition. Following an internal investigation, the manufacturer has discovered the potential for silicone compounds from the SureFold packaging gasket to migrate onto the lens surface (unpublished data, Bausch and Lomb Surgical, Rochester, NY, USA). It is thought that these compounds may act as a catalyst for the nucleation of calcium ions onto the surface of the lens. Bausch and Lomb has therefore decided to replace the current gasket material with a non-silicone material as soon as possible.
We also note that in all our cases except two where opacification was present, a dispersive viscoelastic was used during surgery (Viscoat®). In the remaining two cases, the viscoelastic used was not documented. However, in two eyes where the Hydroview lens used in the fellow eyes remained clear, a cohesive (Healonid®) and a dispersive (Viscoat®) viscoelastic were used. It is worth noting that in the past, two studies reported corneal calcium-phosphate precipitates related to the phosphate buffer concentration in a viscoelastic which prompted the manufacturer to reduce it.11,12 However, not all reported cases4,6 of Hydroview opacification involved the use of a specific viscoelastic and even in the same patient in our series (case 19) and using similar materials on both eyes, only one eye developed late opacification.
In a retrospective case/control study carried out by Bausch and Lomb at centres with the highest reported rates of calcification, there was a trend towards an association with diabetic retinopathy and other ocular surgeries, but this did not reach statistical significance (unpublished data, Bausch and Lomb Surgical, Rochester, NY, USA). In our series, seven patients were either diabetics or underwent combined surgery, all but one were significantly involved requiring explantation. The disturbance of blood/eye barrier may be the underlying factor in promoting calcium deposition in both situations.
It seems therefore that opacification of Hydroview IOL is a delayed phenomenon which is multifactorial in origin. Only IOL delivered in the SureFold packaging is liable to late calcium deposition. The phenomenon may also be related to the type of viscoelastic used during surgery and other patient-specific factors such as diabetes or other eye surgery. The new silicone-free gasket should eliminate this unfortunate and unexpected problem though careful evaluation and prospective surveillance is undoubtedly necessary.
References
Mamalis N . Complications of foldable intraocular lenses requiring explantation or secondary intervention—1998 survey. J Cataract Refract Surg 2000; 26: 766–772
Parkin B, Pitts-Crick M . Opacification of silicone intraocular implant requiring lens exchange (letter). Eye 2000; 14: 794–795
Chang BYP, Davey KG, Gupta M, Hutchinson C . Late clouding of an acrylic intraocular lens following routine phacoemulsification. Eye 1999; 13: 807–808
Werner L, Apple DJ, Escobar-Gomez M, Ohrstrom A, Crayford BB, Bianchi R et al. Postoperative deposition of calcium on the surfaces of a hydrogel intraocular lens. Ophthalmology 2000; 107: 2179–2185
Murray RI . Two cases of late opacification of the hydroview hydrogel intraocular lens. J Cataract Refract Surg 2000; 26: 1272–1273
Sharma TK, Chawdhary S . The opalescence of hydrogel intraocular lens (letter). Eye 2001; 15: 97–98
Stevens A, Chalk BT . Pigments and minerals. In: Bancroft JD, Stevens A (eds). Theory and Practice of Histological Techniques, 4th edn Churchill Livingstone: London 1996
Olson RJ, Caldwell KD, Crandall AS et al. Intraocular crystallization on the intraocular lens surface. Am J Ophthalmol 1998; 126: 177–184
Bucher PJM, Büchi ER, Daicker BC . Dystrophic calcification of an implanted hydroxyethylmethacrylate intraocular lens. Arch Ophthalmol 1995; 113: 1431–1435
Tripathi RC, Tripathi BJ, Silverman RA, Rao GN . Contact lens deposits and spoilage: identification and management (review). Int Ophthalmol Clin 1991; 31: 91–120
Ullman S, Lichtenstein SB, Heerlein K . Corneal opacities secondary to viscoat®. J Cataract Refract Surg 1986; 12: 489–492
Binder PS, Deg JK, Kohl FS . Calcific band keratopathy after intraocular chondroitin sulfate. Arch Ophthalmol 1987; 105: 1243–1247
Acknowledgements
The authors have no financial interests in any of the products in this article.
This article was presented in part as a poster at the Royal College of Ophthalmologists Annual Meeting in Birmingham, May 2001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Habib, N., Freegard, T., Gock, G. et al. Late surface opacification of Hydroview® intraocular lenses. Eye 16, 69–74 (2002). https://doi.org/10.1038/sj.eye.6700069
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6700069