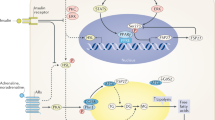
Abstract
Adipocytes isolated from cachectic mice bearing the MAC 16 tumour showed over a 3-fold increase in lipolytic response to both low concentrations of isoprenaline and a tumour-derived lipid mobilizing factor (LMF). This was reflected by an enhanced stimulation of adenylate cyclase in plasma membrane fractions of adipocytes in the presence of both factors. There was no up-regulation of adenylate cyclase in response to forskolin, suggesting that the effect arose from a change in receptor number or G-protein expression. Immunoblotting of adipocyte membranes from mice bearing the MAC16 tumour showed an increased expression of Gαs up to 10% weight loss and a reciprocal decrease in Gα. There was also an increased expression of Gαs and a decrease in Gα in adipose tissue from a patient with cancer-associated weight loss compared with a non-cachectic cancer patient. The changes in G-protein expression were also seen in adipose tissue of normal mice administered pure LMF as well as in 3T3L1 adipocytes in vitro. The changes in G-protein expression induced by LMF were attenuated by the polyunsaturated fatty acid, eicosapentaenoic acid (EPA). This suggests that this tumour-derived lipolytic factor acts to sensitize adipose tissue to lipolytic stimuli, and that this effect is attenuated by EPA, which is known to preserve adipose tissue in cancer cachexia. © 2001 Cancer Research Campaign www.bjcancer.com
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Beck, SA & Tisdale, MJ (1987). Production of lipolytic and proteolytic factors by a murine tumor-producing cachexia in the host. Cancer Res, 47, 5919–5923.
Beck, SA, Smith, KL & Tisdale, MJ (1991). Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res, 51, 6089–6093.
Belsham, GJ, Denton, RM & Tanner, MJA (1980). Use of a novel rapid preparation of fat-cell plasma membranes employing percoll to investigate the effects of insulin and adrenaline on membrane protein phosphorylation within intact fat cells. Biochem J, 192, 457–467.
Carel, JC, Stunff, CL & Condamine, L (1999). Resistance to the lipolytic action of epinephrine: A new feature of protein Gs deficiency. J Clin Endocrinol Metab, 84, 4127–4131.
Costa, G (1963). Cachexia, the metabolic component of neoplastic disease. Progr Exp Tumour Res, 3, 321–369.
Dieudonne, M-N, Pecquery, R, Dausse, J-P & Giudicelli, Y (1993). Regulation of white adipocyte guanine nucleotide binding proteins Gsα and Giα1–2by testosterone in vivo: influence of regional fat distribution. Biochim Biophys Acta, 1176, 123–127.
Gasic, S, Tian, B & Green, A (1999). Tumor necrosis factor α stimulates lipolysis in adipocytes by decreasing Gi protein concentrations. J Biol Chem, 274, 6770–6775.
Green, A, Milligan, G & Dobias, SB (1992). Gi Down-regulation as a mechanism for heterologous desensitization in adipocytes. J Biol Chem, 267, 3223–3229.
Groundwater, P, Beck, SA, Barton, C, Adamson, C, Ferrier, IN & Tisdale, MJ (1990). Alteration of serum and urinary lipolytic activity with weight loss in cachectic cancer patients. Br J Cancer, 62, 816–821.
Hinsch, KD, Rosenthal, W, Spicher, K, Binder, T, Gauespohl, H, Frank, R, Scultz, G & Joost, HG (1988). Adipose plasma membranes contain two Gi subtypes but are devoid of Go. FEBS Lett, 238, 191–196.
Hirai, K, Hussey, HJ, Barber, MD, Price, SA & Tisdale, MJ (1998). Biological evaluation of a lipid mobilizing factor (LMF) isolated from the urine of cancer patients. Cancer Res, 58, 2359–2365.
Klein, S & Wolfe, RR (1990). Whole body lipolysis and triglyceride-fatty acid cycling in cachectic patients with oesophageal cancer. J Clin Invest, 86, 1403–1408.
Levitzki, A (1987). Regulation of adenylate cyclase by hormones and G-proteins. Fed Eur Biochem Soc Lett, 211, 113–118.
Milligan, G & Saggerson, ED (1990). Concurrent up-regulation of guanine-nucleotide-binding proteins Gi1α, Gi2α and Gi3α in adipocytes of hypothyroid rats. Biochem J, 270, 765–769.
Price, SA & Tisdale, MJ (1998). Mechanism of inhibition of a tumor lipid-mobilizing factor by eicosapentaenoic acid. Cancer Res, 58, 4827–4831.
Salomon, Y, Londos, C & Rodbell, M (1974). A highly sensitive adenylate cyclase assay. Anal Biochem, 58, 541–548.
Thompson, MP, Koons, JE, Tan, ETH & Grigor, MR (1981). Modified lipoprotein lipase activities, rates of lipogenesis and lipolysis as factors leading to lipid depletion in C57BL mice bearing the preputial gland tumor, ESR-586. Cancer Res, 41, 3228–3232.
Thompson, MP, Cooper, ST, Parry, BR & Tuckey, JA (1993). Increased expression of the mRNA for the hormone-sensitive lipase in adipose tissue of cancer patients. Biochim Biophys Acta, 1180, 236–241.
Todorov, PT, McDevitt, TM, Meyer, DJ, Ueyama, H, Ohkubo, I & Tisdale, MJ (1998). Purification and characterization of a tumor lipid mobilizing factor (LMF). Cancer Res, 58, 2353–2358.
Wieland, O (1974). Glycerol UV method. Methods of Enzymatic Analysis, Bergmeyer HU (ed) 1404–1409, Academic Press: London
Wigmore, SJ, Ross, JA, Falconer, JS, Plester, CE, Tisdale, MJ, Carter, DC & Fearon, KCH (1996). The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition, 12, S27–S30.
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Islam-Ali, B., Khan, S., Price, S. et al. Modulation of adipocyte G-protein expression in cancer cachexia by a lipid-mobilizing factor (LMF). Br J Cancer 85, 758–763 (2001). https://doi.org/10.1054/bjoc.2001.1992
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1054/bjoc.2001.1992
Keywords
This article is cited by
-
Significance of preoperative evaluation of modified advanced lung cancer inflammation index for patients with resectable non-small cell lung cancer
General Thoracic and Cardiovascular Surgery (2024)
-
The burning furnace: Alteration in lipid metabolism in cancer-associated cachexia
Molecular and Cellular Biochemistry (2022)
-
Preclinical and clinical studies on cancer-associated cachexia
Frontiers in Biology (2018)
-
Cachexia in patients with oesophageal cancer
Nature Reviews Clinical Oncology (2016)
-
Proteomic profiling of the hypothalamus in a mouse model of cancer-induced anorexia-cachexia
British Journal of Cancer (2013)